Splenectomy, or partial or complete removal of the spleen, has been known to confer a long-term increased risk of infection since 1919.1 The most feared complication is overwhelming postsplenectomy infection, a bloodstream infection, usually due to pneumococcus, with a 50% mortality rate.2 Despite an extensive literature on postsplenectomy infection, it remains difficult for clinicians to provide an individualised assessment of that risk for an asplenic patient at the bedside. Since postsplenectomy infections are relatively rare events, large patient populations are needed to estimate incidence reliably. Furthermore, the relatively short follow-up used in many studies may underestimate the frequency of infections. Although most infections occur within the first 2 years after splenectomy, up to a third may be manifested at least 5 years later.3 In addition, the frequency and types of infections other than postsplenectomy infection, pneumococcal disease and meningitis are poorly documented.4 The aim of this study was to determine outcomes after splenectomy by studying a group of patients who underwent splenectomy over an 8-year period.
The Victorian Admitted Episodes Dataset is maintained by the Victorian Department of Health and is based on hospital data compiled by all public and private hospitals in the state. The dataset contains demographic and clinical information on each episode of patient care, with information coded according to the International statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM).5 Death registration data are compiled by the Victorian Registry of Births, Deaths and Marriages.
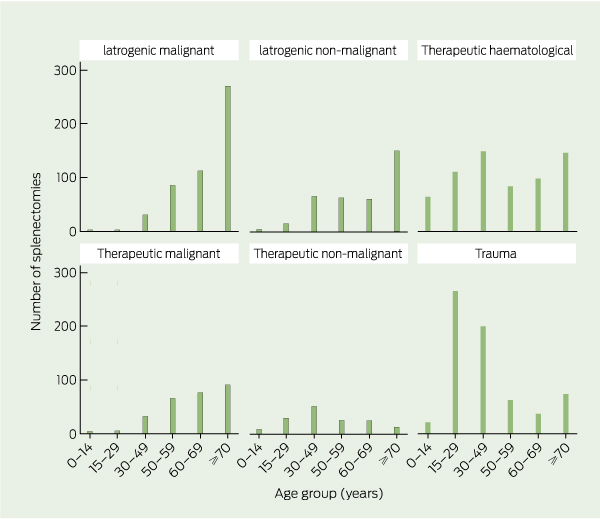
A total of 2574 patients underwent splenectomy in Victoria over the 8-year study period from 1 July 1998 to 31 December 2006. There were 8648 person-years follow-up with a mean follow-up of 3 years, 5 months per person. Splenectomised patient demographics are shown in Box 1. There were 102 paediatric cases (< 15 years of age). These were excluded from subsequent analysis as not being representative of the overall group, leaving 2472 adult splenectomies for analysis. The most common reasons for splenectomy were trauma (25.7%) and therapeutic haematological indications (23.6%).
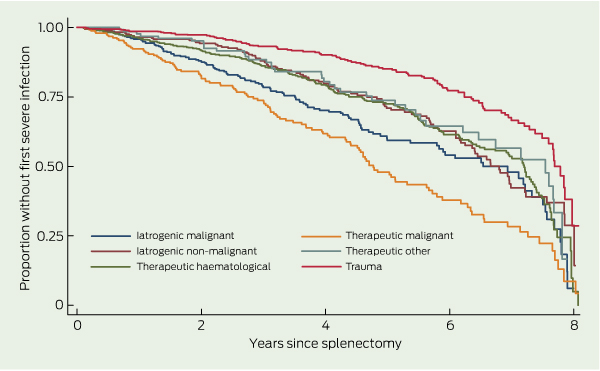
The rate of FSI differed significantly according to age, sex and indication for splenectomy, with female sex, age < 50 years and trauma as the indication for the splenectomy having significantly lower unadjusted rates of first infection. These relationships did not change after adjustment (Box 2).
Eighty-three per cent of infections fell into nine categorised body sites; the remainder were uncategorisable (Box 3). Respiratory infections and sepsis (proven or presumed bloodstream infections) were the most common sites of infection. Gram-negative infections represented the most frequent causative organism group, accounting for 698 (51%) of bacterial pathogens. Staphylococcus aureus accounted for 414 (31%) of infections. Streptococcus pneumoniae, meningococcal species and Haemophilus influenzae accounted for 7 (5%), 4 (3%) and 10 (7%) infections, respectively. Compared with splenectomised trauma patients, the adjusted HR of first severe sepsis was 16.5 (95% CI, 6.1–44.6; P ≤ 0.001) for patients splenectomised for therapeutic malignancy; 6.2 (95% CI, 2.4–16.3; P < 0.001) for patients splenectomised for therapeutic haematological indications; 4.9 (95% CI, 1.7–14.3; P = 0.003) for patients splenectomised for iatrogenic malignancy; and 2.9 (95% CI, 0.7–12.2; P = 0.149) for those splenectomised for therapeutic other indications (Box 4).
The rate of FSI after splenectomy declined over time. The highest risk of infection was seen within the first 2 years after splenectomy, with 25% of all FSIs occurring during this time, 50% of infections by 3.7 years, 75% by 5.7 years, and 95% by 7.6 years. The cumulative incidence at 3, 6, 12 and 24 months was 73.4, 53.9, 38.3 and 22.1 per 100 person-years, respectively. However, first infections did continue to occur late, with the cumulative incidence at 8 years being 7.8 per 100 person-years (Box 5).
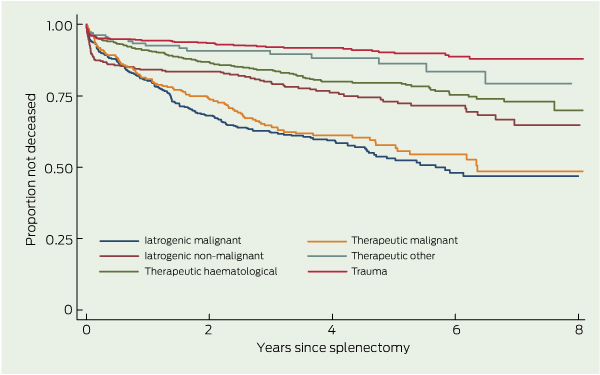
Overall, 562 splenectomised patients (22.7%) died during the follow-up period, with 395 (15.3%) dying within the first 2 years (Box 6). The median survival for patients who died during the follow-up period after splenectomy was 3.1 years (interquartile range [IQR], 1.3–5.3). There was an increased adjusted hazard of death for individuals aged ≥ 50 years compared with those aged < 50 years (HR, 4.8; 95% CI, 3.5–6.3). Women had a decreased hazard of death compared with men (HR, 0.8; 95% CI, 0.6–0.9) (Box 7).
Vaccination, education and antibiotic prophylaxis are recognised as reducing the risk of postsplenectomy sepsis and death.6 Although the Australasian Society for Infectious Diseases has published guidelines,7 the appropriate duration of antibiotic prophylaxis in otherwise immunocompetent hosts remains ambiguous. Consequently, the questions remain of who should receive antibiotic prophylaxis and for how long? Committing patients to lifelong antibiotics is difficult; therefore, more information on how to tailor therapy can assist decision making. Our study has identified patient groups at highest risk for adverse outcomes and can be used to guide management decisions.
Based on one of the largest population-based cohorts of splenectomised patients in the literature, our results show that 26% of splenectomised patients had at least one severe infection requiring hospitalisation during the period of observation, with these occurring at a mean of 3.5 years after splenectomy. Our rate of postsplenectomy infection of 8.0 per 100 person years is consistent with international studies (using the same case-definition for severe infection), which have reported rates of 7.0,8 7.2,9 and 7.710 per 100 person-years. A Western Australian study reported a substantially lower rate of severe infection of 0.4 per 100 person-years, but included only septicaemia, meningitis and pneumococcal pneumonia.11 In contrast, a Danish civil registry system reported a risk of severe infection in the community of 2.0 per 100 person-years.10
A quarter of severe infections occurred in the first 2 years after splenectomy, with the highest risk in the first 6 months, consistent with the findings in other studies.8,12-15 A Danish population-based cohort study reported that 10.2% of infections occurred within 90 days of splenectomy, with a relative risk of 18.1 compared with the general population.10 In our cohort, 31.8% of FSI occurred more than 5 years after splenectomy. Late postsplenectomy sepsis is a less well recognised problem,11 but fulminant infection has been documented more than 20 years after splenectomy.11,16,17 Our data support the statement that asplenic patients should be considered to have a risk of developing severe late postsplenectomy infection up to at least 5 years after splenectomy.
The rates of infection were lowest among patients splenectomised for trauma and highest among those splenectomised for malignant disease, in keeping with other literature.8,9,12,13 Notably, trauma patients may have a partial protective effect from splenosis, owing to self-implanted remnants of the traumatised spleen.18 Partly because of the problems associated with using an administrative dataset, it is difficult to determine whether infection or death was due to the splenectomised state or to the intrinsic risk conferred by the underlying disease process as well as immunosuppressive therapies. This represents an important limitation of our study.
Late in the period of observation (2003) the Victorian Spleen Registry was established. One of its major roles is in providing education to splenectomised patients.19 The median incidence of FSI in the first year after splenectomy was higher than in the 4 years before the Registry began, compared with the 2 years after introduction. However, the short follow-up periods make these data difficult to interpret and unlikely to have a major influence over our findings.
Our study found that encapsulated organisms were less common than gram-negative organisms and S. aureus. It is difficult to determine if this represents uptake of vaccination and antibiotic prophylaxis in Victoria or a changing pattern of postsplenectomy infections. Targeted studies are warranted to determine the microbiology of infecting organisms and whether penicillin remains the most appropriate agent for antibiotic prophylaxis. Prospective studies are needed to analyse infectious outcome data relating to the use of prophylaxis, both with antibiotics and vaccinations. The use of such data to develop a risk assessment tool, similar to the CHADS2 score for anticoagulation in atrial fibrillation,20 and other individualised guidelines for postsplenectomy care would greatly assist clinicians managing asplenic patients.
2 Adjusted rates of first severe infection in splenectomised adults (n = 2472)
* Proportional hazards test, P = 0.2019. † Per 100 person-years. |
|||||||||||||||
4 Adjusted rates of sepsis in splenectomised adults (n = 2472)
* Proportional hazards test, P = 0.3389. † Per 100 person-years. |
|||||||||||||||
Received 17 July 2011, accepted 19 March 2012
- Claire Dendle1,2
- Vijaya Sundararajan2
- Tim Spelman3,4
- Damien Jolley4
- Ian Woolley1,2
- 1 Department of Infectious Diseases, Monash Medical Centre, Southern Health, Melbourne, VIC.
- 2 Department of Medicine, Monash University, Melbourne, VIC.
- 3 Centre for Population Health, Burnet Institute, Melbourne, VIC.
- 4 School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC.
Our work was supported by a grant from the Victorian Trauma Foundation.
No relevant disclosures.
- 1. Morris DH, Bullock FD. The importance of the spleen in resistance to infection. Ann Surg 1919; 70: 513-521.
- 2. Standage BA, Goss JC. Outcome and sepsis after splenectomy in adults. Am J Surg 1982; 143: 545-548.
- 3. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect 2001; 43: 182-186.
- 4. Green JB, Shackford SR, Sise MJ, Powell RW. Postsplenectomy sepsis in pediatric patients following splenectomy for trauma: a proposal for a multi-institutional study. J Pediatr Surg 1986; 21: 1084-1086.
- 5. Australian Government Department of Health. The international statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM). 4th ed. Sydney: National Centre for Classification in Health, 2004
- 6. El-Alfy MS, El-Sayed MH. Overwhelming postsplenectomy infection: is quality of patient knowledge enough for prevention? Hematol J 2004; 5: 77-80.
- 7. Spelman D, Buttery J, Daley A, et al; Australasian Society for Infectious Diseases. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J 2008; 38: 349-356.
- 8. Kyaw MH, Holmes EM, Toolis F, et al. Evaluation of severe infection and survival after splenectomy. Am J Med 2006; 119: 276.e1-7.
- 9. Schwartz PE, Sterioff S, Mucha P, et al. Postsplenectomy sepsis and mortality in adults. JAMA 1982; 248: 2279-2283.
- 10. Thomsen RW, Schoonen WM, Farkas DK, et al. Risk for hospital contact with infection in patients with splenectomy: a population-based cohort study. Ann Intern Med 2009; 151: 546-555.
- 11. Cullingford GL, Watkins DN, Watts AD, Mallon DF. Severe late postsplenectomy infection. Br J Surg 1991; 78: 716-721.
- 12. Yong M, Thomsen RW, Schoonen WM, et al. Mortality risk in splenectomised patients: a Danish population-based cohort study. Eur J Intern Med 2010; 21: 12-16.
- 13. Ejstrud P, Kristensen B, Hansen JB, et al. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand J Infect Dis 2000; 32: 521-525.
- 14. Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg 1991; 78: 1031-1038.
- 15. Deodhar HA, Marshall RJ, Barnes JN. Increased risk of sepsis after splenectomy. BMJ 1993; 307: 1408-1409.
- 16. Waghorn DJ, Mayon-White RT. A study of 42 episodes of overwhelming post-splenectomy infection: is current guidance for asplenic individuals being followed? J Infect 1997; 35: 289-294.
- 17. Ellison EC, Fabri PJ. Complications of splenectomy. Etiology, prevention, and management. Surg Clin North Am 1983; 63: 1313-1330.
- 18. Rice HM, James PD. Ectopic splenic tissue failed to prevent fatal pneumococcal septicaemia after splenectomy for trauma. Lancet 1980; 1: 565-566.
- 19. Denholm JT, Jones PA, Spelman DW, et al. Spleen registry may help reduce the incidence of overwhelming postsplenectomy infection in Victoria. Med J Aust 2010; 192: 49-50. <MJA full text>
- 20. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133 (6 Suppl): 546S-592S.





Abstract
Objective: To determine the risk and timing of a broad range of infective outcomes and mortality after splenectomy.
Design, setting and participants: Analysis of a non-identifiable linked hospital discharge administrative dataset for splenectomy cases between July 1998 and December 2006 in Victoria, Australia.
Main outcome measures: Age, sex, indication for splenectomy, infectious events and death. Patients splenectomised for trauma were compared with patients splenectomised for other indications. Infectious risk was established using Cox proportional hazards models.
Results: A total of 2574 patients underwent splenectomy (with 8648 person-years follow-up). Paediatric cases were excluded, leaving 2472 adult cases for analysis. The most common reasons for splenectomy were trauma (635 [25.7%]) and therapeutic haematological indications (583 [23.6%]). After splenectomy, 644 adult patients (26.0%) had a severe infection, with a rate of 8.0 per 100 person-years (95% CI, 7.2–8.4). The risk of severe infection was highest among patients aged ≥ 50 years (1.9 per 100 person-years; 95% CI, 1.6–2.7) and those splenectomised for malignancy (14.2 per 100 person-years; 95% CI, 11.8–17.1). Gram-negative infections represented the most frequent causative organism group accounting for 698 (51%) of bacterial pathogens. Staphylococcus aureus was the second most common causative organism.
Conclusion: The incidence of severe infection and all-cause mortality differed according to age and underlying reason for splenectomy, and was highest among the elderly and those with malignancy, and was lowest among trauma patients. This highlights the need for targeted prevention programs.