Glycaemic control in hospitalised patients has become a major therapeutic focus. The prevalence of diabetes in Australian hospital inpatients is about 12%, with another 11% having undiagnosed diabetes.1 Higher glucose concentrations in hospitalised patients are independently associated with increased morbidity and mortality in a broad array of disease states.2-7 Although not all studies report a benefit, lowering blood glucose levels (BGLs) may reduce morbidity and mortality in some patient groups.8-11 Therefore, it is recommended that hyperglycaemia be treated in most hospitalised patients.12
Subcutaneous sliding-scale insulin (SSI) has historically been the mainstay of hyperglycaemia management for ward-based patients. However, SSI does not provide good glycaemic control.13-15 Recent randomised controlled trials (RCTs) found that an approach using basal–bolus insulin (BBI) produced a lower mean BGL in hospitalised patients with type 2 diabetes compared with SSI.16,17 In surgical patients, BBI therapy was also associated with reduced morbidity.17 BBI is now recommended therapy for patients with hyperglycaemia on the general ward, although its efficacy and safety outside a clinical trial environment are based on limited evidence.12
In real-world clinical practice, drug use and subsequent outcomes often deviate from the carefully scripted nature of RCTs.18 Strict inclusion and exclusion criteria and close monitoring in RCTs may contribute to failure to replicate results in routine practice.19 For example, spironolactone for treating heart failure did not cause significant hyperkalaemia in a landmark RCT,20 but did under routine clinical conditions when used in a less selective fashion.21 Although the recent RCTs provide proof of concept for BBI’s superiority to SSI, they recruited a selective group of patients and involved a daily round by an endocrinologist.16,17 To determine whether BBI is superior to SSI when administered by non-supervised junior medical staff working in a normal clinical environment, we compared glycaemic control in inpatients after a hospital-wide change from using SSI to using a BBI protocol.
There were no statistically significant differences in age, sex, weight, length of stay, HbA1c level, creatinine clearance, vomiting, or intravenous glucose administration between the two treatment groups (Box 1). Patients receiving BBI had a higher BMI, higher American Society of Anesthesiologists score (for surgical patients), more comorbidities and active infections, and were more likely to have been prior insulin users and less likely to be undergoing surgery.
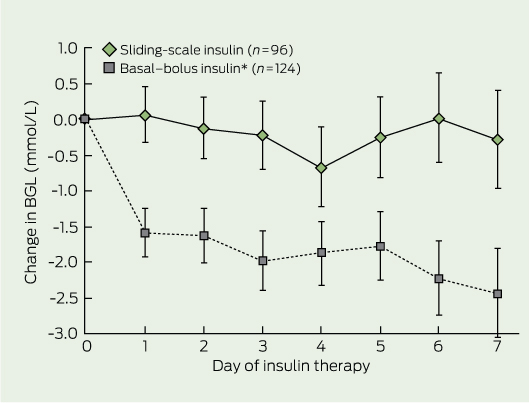
There was no significant difference in mean baseline BGL in patients receiving BBI and SSI (Box 1). Mean ± SD daily BGL in patients receiving BBI was 1.6 ± 3.7 mmol/L lower than baseline BGL (P < 0.001) after the first full day of insulin therapy, and it remained lower than baseline throughout the study (2.4 ± 4.8 mmol/L lower at Day 7; P < 0.001) (Box 2). In contrast, there was no statistically significant change in mean daily BGL from baseline on any given day for patients receiving SSI.
The percentage of BGLs in the desired range (4–10 mmol/L) was significantly greater in patients receiving BBI than in those receiving SSI (56.0% v 50.3%; P < 0.001), as was the percentage of BGLs < 4 mmol/L (3.3% v 1.4%; P < 0.001). However, the percentage of BGLs < 2.8 mmol/L in the two groups was not significantly different (0.3% v 0.5%; P = 0.3). In patients receiving BBI, the incidence of hypoglycaemia was not significantly different across quartiles of insulin dose (Box 3). The percentages of BGLs > 10 mmol/L (40.6% v 48.3%; P < 0.001) and > 20 mmol/L (1.1% v 2.9%; P < 0.001) were significantly lower in patients receiving BBI than in those receiving SSI.
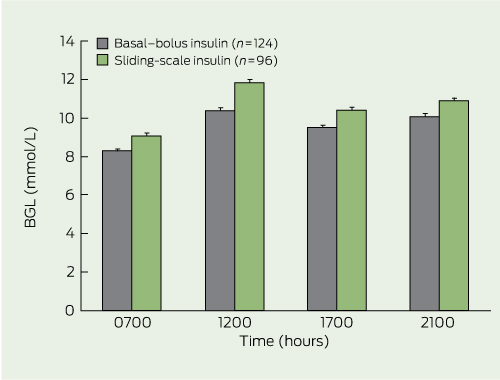
The mean BGL was 0.8–1.4 mmol/L lower in patients receiving BBI than those administered SSI at each designated measurement time (P < 0.001 for each time point) (Box 4).
This study demonstrates that use of a hospital BBI protocol by junior medical staff to treat hyperglycaemia resulted in lower mean daily BGLs in non-critical medical and surgical inpatients for up to 7 days compared with empirical management with SSI. The improvement was seen within 24 hours of starting BBI and was evident at all BGL measurement times. Patients receiving BBI had an increased incidence of hypoglycaemia, but not severe hypoglycaemia. These findings are similar to those reported under rigorous study conditions and suggest that BBI is effective, safe, sustainable and has reproducible outcomes outside the formal study setting across a wide range of patients.16,17 Although our study did not test an optimal SSI regimen and therefore does not provide definitive proof that BBI is superior to SSI, our data extend the published data by showing that BBI appears to be superior to SSI in real-world clinical practice.
There are several barriers to implementing BBI as “routine therapy” in hospitalised patients. These include an increased nursing workload, scepticism regarding the benefits of good glycaemic control, fear of hypoglycaemia, lack of specialist input, and an inadequate knowledge of diabetes on the part of the junior medical and nursing staff responsible for applying the protocol.22 However, our study demonstrates that introducing BBI with limited education of junior medical and nursing staff reduces hyperglycaemia in hospitalised patients.
Our pragmatic study design has limitations, but provides complementary information to the RCTs.16,17 The two study groups were not matched for several variables, but this was accounted for by using a random-effects regression analysis which demonstrated that BBI lowered mean daily BGL independently of other variables.
The lower mean BGL in patients receiving BBI reflects the higher doses of insulin administered to this group. Patients receiving SSI had a daily insulin dose directly from SSI of only 8–11 units, while the mean daily administered BBI dose was about 50 units, consistent with the RCTs.16,17 However, our study differed from the previous studies in that SSI was “add on” therapy to usual care in most patients, which will have improved their glycaemic control. In our experience, SSI is often combined with usual therapy.
Tighter glycaemic control is associated with an increased rate of hypoglycaemia.16,17 In our study, patients administered BBI experienced increased hypoglycaemia (BGL < 4.0 mmol/L) but not severe hypoglycaemia (BGL < 2.8 mmol/L). In outpatients with type 2 diabetes, a BGL < 2.8 mmol/L is associated with increased cardiovascular morbidity and mortality, while milder hypoglycaemia is not.23 It is unknown if this threshold applies to hospitalised patients, as the consequences of hypoglycaemia may be more severe in acutely unwell patients (eg, sympathetic stimulation in a patient with unstable cardiac disease). Nevertheless, our results suggest hypoglycaemia in patients receiving BBI is likely to be at the milder end of the spectrum and, as such, less likely to adversely affect patient outcomes.
The assessment of hypoglycaemia in our study is subject to limitations, as it was based on finger-prick capillary BGLs, which are less accurate than laboratory glucose measurements. Furthermore, it is unknown how many episodes were symptomatic and how many hypoglycaemic episodes occurred at other times of the day. Further, the period during which data were collected differed for the two groups, but both insulin regimens were assessed after the seminal studies demonstrating benefit from tight outpatient and inpatient glycaemic control.10,24 As with all observational studies, certain variables affecting glycaemic control in the two groups may have gone unmeasured.
2 Mean change in blood glucose level (BGL) from baseline BGL in the two insulin therapy groups
 | |||||||||||||||
|
* P < 0.001 v baseline BGL for all days of basal–bolus insulin therapy. Bars indicate SE. | |||||||||||||||
Received 6 July 2011, accepted 6 November 2011
- Greg W Roberts1,2
- Norma Aguilar-Loza3
- Adrian Esterman4
- Morton G Burt2,3
- Stephen N Stranks2,3
- 1 Pharmacy Department, Repatriation General Hospital, Adelaide, SA.
- 2 School of Medicine, Flinders University, Adelaide, SA.
- 3 Southern Adelaide Diabetes and Endocrine Services, Repatriation General Hospital, Adelaide, SA.
- 4 Sansom Institute of Health Service Research and School of Nursing and Midwifery, University of South Australia, Adelaide, SA.
This study was supported by a grant from Sanofi-Aventis to Greg Roberts and Stephen Stranks. However, Sanofi-Aventis played no role in study design, data analysis or drafting of the manuscript.
No relevant disclosures.
- 1. Valentine NA, Alhawassi TM, Roberts GW, et al. Detecting undiagnosed diabetes using glycated haemoglobin: an automated screening test in hospitalised patients. Med J Aust 2011; 194: 160-164. <MJA full text>
- 2. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773-778.
- 3. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003; 78: 1471-1478.
- 4. Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med 2006; 166: 1613-1619.
- 5. Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 2006; 61: 284-289.
- 6. Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63: 356-361.
- 7. Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426-2432.
- 8. Finfer S, Chittock DR, Su SY, et al; NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283-1297.
- 9. Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57-65.
- 10. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359-1367.
- 11. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999; 67: 352-360.
- 12. American Diabetes Association. Executive summary: standards of medical care in diabetes — 2011. Diabetes Care 2011; 34 Suppl 1: S4-S10.
- 13. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med 1997; 157: 545-552.
- 14. Golightly LK, Jones MA, Hamamura DH, et al. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy 2006; 26: 1421-1432.
- 15. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm 2004; 61: 1611-1614.
- 16. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30: 2181-2186.
- 17. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34: 256-261.
- 18. Brass EP. The gap between clinical trials and clinical practice: the use of pragmatic clinical trials to inform regulatory decision making. Clin Pharmacol Ther 2010; 87: 351-355.
- 19. Ware JH, Hamel MB. Pragmatic trials — guides to better patient care? N Engl J Med 2011; 364: 1685-1687.
- 20. Pitt B, Zannad F, Remme WJ, et al; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709-717.
- 21. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543-551.
- 22. Kitabchi AE, Nyenwe E. Sliding-scale insulin: more evidence needed before final exit? Diabetes Care 2007; 30: 2409-2410.
- 23. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410-1418.
- 24. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853.





Abstract
Objective: To determine if the improvement in inpatient glycaemic control observed with basal–bolus insulin (BBI) over sliding-scale insulin (SSI) in the formal study setting translates to routine clinical conditions.
Design, setting and patients: Cross-sectional study in which capillary blood glucose levels (BGLs) were prospectively measured four times daily for up to 8 days in 124 patients with type 2 diabetes admitted to a tertiary teaching hospital and treated with BBI between November 2008 and May 2010. Data from the BBI treatment group were compared with retrospective data from 96 patients treated with SSI between June 2001 and May 2006.
Main outcome measures: Mean daily BGL; independent effect of insulin regimen on mean daily BGL.
Results: Mean baseline BGL was not significantly different in patients receiving BBI and SSI (mean ± SD, 11.3 ± 4.1 v 10.6 ± 4.3 mmol/L; P = 0.23). After the first full day of therapy, mean daily BGL for patients receiving BBI was 1.6 ± 3.7 mmol/L lower than baseline BGL, and it remained 1.6–2.4 mmol/L lower than baseline throughout the study (P < 0.001). In contrast, there was no significant change in BGL for patients receiving SSI. Random effects regression analysis indicated that BBI was associated with a significantly lower mean daily BGL than SSI, independent of other variables (P < 0.001). The incidence of hypoglycaemia (BGL < 4 mmol/L) was significantly greater in patients receiving BBI than SSI (3.3% v 1.4%; P < 0.001), but there was no significant difference for severe hypoglycaemia (BGL < 2.8 mmol/L) (0.3 v 0.5%; P = 0.3).
Conclusions: Under routine clinical conditions, BBI is effective and safe across a range of patients and appears to be superior to SSI. Clinical improvements reflected those seen in a strict formal study setting.