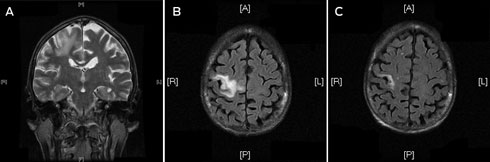
A frail 68-year-old woman of European ancestry presented to the emergency department with partial seizures of her left hand and mild left hemiparesis. Magnetic resonance imaging (MRI) scans of the brain revealed a non-enhancing lesion in the right precentral gyrus that was thought to represent infarction (Box, A and B).
After surgery, the patient began highly active antiretroviral therapy (HAART) with a regimen comprising Trizivir (abacavir, lamivudine and zidovudine) and ritonavir-boosted indinavir. She experienced symptomatic improvement, weight gain and reduced partial seizures within a few weeks. The plasma HIV viral load was undetectable within 8 weeks of commencing HAART and a repeat MRI scan at that time confirmed a significant reduction in size of the cerebral lesion (Box, C).
PML is a demyelinating disease of the CNS caused by JCV, a human polyomavirus. The condition generally occurs in the setting of prolonged immunosuppression among individuals with decreased cell-mediated immunity, such as HIV infection.1 In the pre-HAART era, the prognosis for PML was very poor, with median survival times no greater than 6 months.2 With the advent of HAART, the survival time has improved to 15 months or more, but mortality rates can still be as high as 30% to 50%.3 PML has also been documented among patients who receive certain immunosuppressive drugs, such as fludarabine, rituximab, corticosteroids,2 and shortly after the introduction of HAART as a form of immune-reconstitution inflammatory syndrome.4 In HIV infection, HAART is the only therapeutic option for PML, but the efficacy of these agents in controlling JCV replication in the CNS is variable and clinical response is not uniform. Antiretroviral agents with good CNS penetration have been used to optimise therapy for patients with PML.3
Previous studies have suggested the mean time to AIDS diagnosis from HIV acquisition after transfusion was 7 years,5 although a cohort of “non-progressors” who acquired a variant strain of HIV containing the nef gene deletion has been well documented.6 Our patient had delayed progression to AIDS, even in the absence of HAART, a clinical pattern that has been linked to several other genetic factors, including chemokine coreceptor type 5 (CCR5) status.7, 8 CCR5 is a coreceptor required for HIV to enter T cells and macrophages. Homozygosity for the Δ32 gene deletion in the CCR5 gene (CCR5-Δ32) is associated with resistance to HIV infection (although people can still be infected with T-tropic strains of the virus, which use the CXC chemokine receptor type 4 for cell entry), whereas heterozygosity confers delayed progression to disease. Our patient was confirmed as being heterozygous for CCR5-Δ32. In the United States, the frequency of the allele is 11% among white people and 1.7% among black people.8 Targeting the CCR5 receptor to interrupt HIV transmission offers new therapeutic possibilities. Maraviroc, a CCR5 inhibitor, has been introduced with success when used as part of a HAART regimen for patients with R5 (M-tropic) virus.9 Transplantation of stem cells from a donor homozygous for CCR5-Δ32 to a patient with acute myeloid leukaemia and HIV infection resulted in continued virological suppression after transplantation in the transplant recipient and discontinuation of HAART.10
One approach to help identify individuals who are unaware that they have HIV infection is the “opt-out” HIV testing strategy proposed by the US Centers for Disease Control and Prevention in 2006.11 All people aged 13–64 years in health care settings would have routine HIV testing. Importantly, pretest counselling and signed consent would not be required, and the test would be performed unless the patient declined. Patients with known risks for HIV infection should be tested annually.11 This testing strategy would allow undiagnosed patients earlier access to medical care with an anticipated reduction in HIV transmission and infection related morbidity and mortality.12
The World Health Organization endorsed these recommendations in 2007, expanding the scope of uptake to the developing world.13 Drawbacks to this approach include concerns about stigmatisation and discrimination of individuals with HIV,14 and increased cost implications15 for health authorities from increased test numbers, including confirmatory immunoblot assays, and a requirement for more expertise to interpret true and indeterminate results.
- 1. Demeter LM. JC, BK, and other polyomaviruses; progressive multifocal leukoencephalopathy. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Churchill Livingstone, 2009.
- 2. Weber T. Progressive multifocal leukoencephalopathy. Neurol Clin 2008; 26: 833-854.
- 3. Falcó V, Olmo M, del Saz SV, et al. Influence of HAART on clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicentre study. J Acquir Immune Defic Syndr 2008; 49: 26-31.
- 4. Safdar A, Rubocki RJ, Horvath JA, et al. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis 2002; 35: 1250-1257.
- 5. Kopec-Schrader E, Tindall B, Learmont J, et al. Development of AIDS in people with transfusion-acquired HIV infection. AIDS 1993; 7: 1009-1013.
- 6. Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med 1999; 340: 1715-1722.
- 7. Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 1: cellular and humoral immune responses. Ann Intern Med 2001; 134: 761-776.
- 8. Hogan CM, Hammer SM. Host determinants in HIV infection and disease. part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med 2001; 134: 978-996.
- 9. Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008; 359: 1429-1441.
- 10. Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692-698.
- 11. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents and pregnant women in health care settings. MMWR Recomm Rep 2006; 55 (RR-14): 1-17.
- 12. Bartlett JG, Branson BM, Fenton K, et al. Opt-out testing for human immunodeficiency virus in the United States. Progress and challenges. JAMA 2008; 300: 945-951.
- 13. Bassett IV, Walensky RP. Integrating HIV screening into routine health care in resource limited settings. Clin Infect Dis 2010; 50 Suppl 3: S77-S84.
- 14. Dodds C, Keogh P, Chime O, et al. Outsider status: stigma and discrimination experienced by gay men and African people with HIV. London: Sigma Research, 2004. http://www.sigmaresearch.org.uk/files/report2004f.pdf (accessed Nov 2010).
- 15. Holtgrave DR. Costs and consequences of the US Centers for Disease Control and Prevention’s recommendations for opt-out HIV testing. PLoS Med 2007; 4: e194.





We thank Dr Janice Brewer, Senior Pathologist from the Department of Anatomical Pathology at Royal North Shore Hospital, and Dr Michael Buckland, Honorary Associate of Pathology at the University of Sydney based at the Department of Neuropathology, Royal Prince Alfred Hospital for providing expert histopathological assessment.
No relevant disclosures.