Over the past decade, percutaneous coronary intervention (PCI) has replaced coronary artery bypass graft surgery as the most common coronary revascularisation strategy for treating coronary artery disease in Australia.1 Current procedural success rates are high, with improved clinical outcomes as a result of increasing operator experience and technological and pharmacological advances, including potent antiplatelet therapy, aggressive secondary prevention and drug-eluting stents (DES).2
Uptake of DES was rapid after their introduction in 2003, with use as high as 90% in 2004.3,4 Initial enthusiasm was tempered by concerns over the long-term safety of DES, associated with the potential increased risk of late (> 30 days) and very late (> 12 months) stent thrombosis, myocardial infarction (MI) and mortality.5-7 This led to a significant fall in the use of DES worldwide from 2006.3 However, the safety of DES up to 5-year follow-up has been confirmed in recent studies.8,9 The impact of this controversy relating to DES highlights the need for accurate PCI outcome data in an era of rapid evolution of device technology.
Little is known about PCI practice and outcome trends in the era of DES in Australia. Previous studies that analysed temporal trends of PCI in Australia predate the introduction of DES and lack detailed clinical and patient information to determine trends in patient risk profile.10,11 We aimed to evaluate PCI practice trends and 12-month outcomes in consecutive patients undergoing PCI using data from a large Australian PCI registry.
The study population consisted of consecutive patients undergoing 9204 PCI procedures between 1 April 2004 and 31 March 2008 that were recorded in the Melbourne Interventional Group (MIG) registry. We divided the data into four yearly periods (starting 1 April and ending 31 March the following year) for analysis.
The MIG registry (http://www.ccretherapeutics.org.au/research/mig) has been previously described.4,12 Demographic, clinical and procedural characteristics for consecutive patients undergoing PCI at seven Australian public tertiary referral hospitals are prospectively recorded on case-report forms using standardised definitions for all fields.
An “opt-out” consent process is used, in which patients are provided with an information sheet that describes the registry and its purpose and explains that routine follow-up will be performed. Patients can call a 1800 free-call number if they do not wish to be included in the registry. This model has been recommended in clinical-quality registries and is currently used in other Australian registries.13-15 The study protocol was approved by the ethics committee in each participating hospital.
An independent audit was conducted at all enrolling sites by an investigator not affiliated with the institution. Fifteen verifiable fields from 5% of patients enrolled from each site were randomly audited and demonstrated an overall accuracy of 96.6%.
The interventional strategy and stent selection were left to the discretion of the operator. In 2003, the Victorian Department of Human Services, with the aid of cardiologists, developed clinical guidelines for use of DES in public hospitals, restricting their use to patients at high risk of restenosis who will theoretically derive the greatest benefit.16 The criteria for using DES included one or more of: diabetes mellitus, small target vessels (≤ 2.5 mm diameter), long lesions (≥ 20 mm), and complex lesions such as chronic total occlusion, in-stent restenosis, and bifurcation and ostial lesions.
The PCI procedure was defined as urgent if it was required during an emergency admission to minimise the chance of further clinical deterioration, including unstable angina, heart failure, acute MI and cardiogenic shock.17 Total stent length was used as a surrogate for target lesion length, and stent diameter for target vessel diameter. Procedural success was defined by a residual stenosis of < 20% in stent procedures with TIMI (Thrombolysis in Myocardial Infarction) 3 flow.
Oral antiplatelet therapy followed the recommendations at the time, based on the original randomised trials of bare-metal stents (BMS) and DES, which used a combination of aspirin and clopidogrel for a minimum of 4 weeks for BMS and 3, 6 or 12 months for DES.18
In-hospital complications were recorded at time of discharge. Major bleeding complication was defined as bleeding that occurred during or after the procedure until discharge, that required transfusion and/or prolonged hospital stay and/or caused a drop in haemoglobin level > 3.0 g/dL.17 Thirty-day and 12-month follow-up was conducted by telephone, and cardiac events including death, MI, target vessel revascularisation (TVR; revascularisation of a previously treated coronary artery) and composite major adverse cardiac events (MACE; consisting of death, MI and TVR) were recorded. Cause of death outside hospital was confirmed with the patient’s primary care physician. Stent thrombosis was classified as early (0–30 days after PCI) or late (31–365 days). Patients’ medical records were reviewed to substantiate recorded events including MI, TVR and major bleeding. Any queries or inconsistencies were adjudicated by the site principal investigator (a cardiologist).
Temporal trends in baseline variables were examined with the linear-by-linear association test for categorical variables and by linear regression for continuous variables. The Kaplan–Meier method was used to estimate event-free survival rates, with log-rank tests used for curve comparisons.
Logistic regression models were used to adjust outcomes for differences across years and to estimate odds ratios (ORs) for adverse outcomes. Variables considered in the univariate analysis were: year of procedure; age; sex; use of DES; diabetes mellitus; hypertension; hyperlipidaemia; smoking history; renal impairment; prior heart failure; recent heart failure; family history of coronary artery disease; peripheral arterial disease; previous PCI; previous coronary artery bypass graft; acute coronary syndrome; cardiogenic shock; stroke; previous MI; use of glycoprotein IIb/IIIa inhibitor; multivessel disease; left main, left anterior descending or right coronary artery treated; bypass graft treated; American College of Cardiology/American Heart Association (ACC/AHA) type B2 and C lesions;19 ostial, bifurcation and restenotic lesions; chronic total occlusion; stent length ≥ 20 mm; stent diameter ≤ 2.5 mm; and intended duration of clopidogrel therapy. Univariate variables with P < 0.1 were included in the multivariate model.
All calculated P values were two-sided, and P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 17.0 for Windows (SPSS Inc, Chicago, Ill, USA).
Baseline characteristics are shown in Box 1. During the study period, the mean age of patients was stable (65 ± 12 years). Prevalences of comorbidities such as hypertension, hyperlipidaemia, peripheral arterial disease and stroke increased from 2004–2005 to 2007–2008 (all P < 0.05).
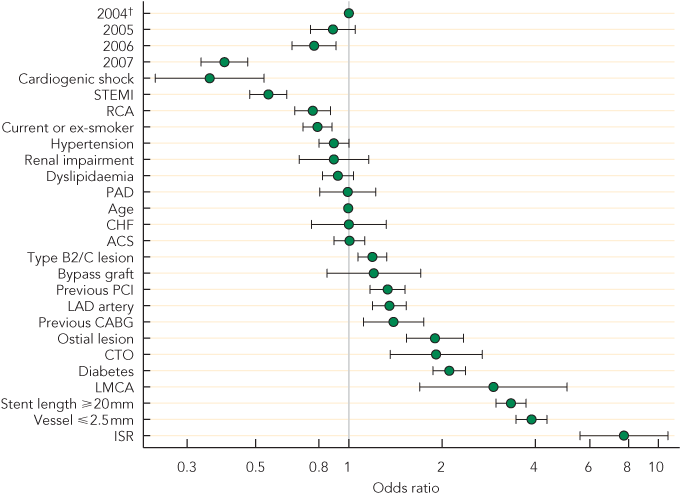
During the study period, there were increasing proportions of urgent procedures, and procedures for ST-elevation MI (STEMI) and MI < 24 hours (all P < 0.01) (Box 2). Other than an increase for the left anterior descending artery (P = 0.03), there were no significant changes in the types of vessels treated. The mean number of lesions treated per procedure declined (P < 0.01). Overall stent use remained high (mean, 94.6%), but use of DES declined from 53.9% in 2004–2005 to 32.0% in 2007–2008 (P < 0.01), despite an increase in complex lesions being treated, such as in-stent restenosis (P = 0.02), chronic total occlusion, bifurcation and ostial lesions (all P < 0.01). The likelihood of receiving DES decreased in successive years and was lowest in patients with cardiogenic shock and STEMI (Box 3). The use of glycoprotein IIb/IIIa inhibitors increased (P < 0.01), consistent with the increased proportion of patients with STEMI and non-STEMI being treated (Box 2). Procedural success remained high across the years (mean, 95.9%).
Despite the fall in use of DES, overall planned clopidogrel therapy of ≥ 12 months increased from 43.4% in 2004–2005 to 58.5% in 2007–2008 (P < 0.01) (Box 4). Planned duration of clopidogrel therapy was significantly longer after DES than BMS, with planned therapy of ≥ 12 months after DES increasing from 54.7% in 2004–2005 to 98.0% in 2007–2008 (P < 0.01), and remaining at about a third for BMS.
Overall in-hospital mortality, MI and emergency coronary artery bypass graft rates remained low and steady (mean, 1.5%, 1.5% and 0.7%, respectively) (Box 5). However, the incidence of major bleeding increased from 1.0% in 2004–2005 to 2.3% in 2007–2008 (P = 0.001). In contrast, rates of stroke decreased (P = 0.03).
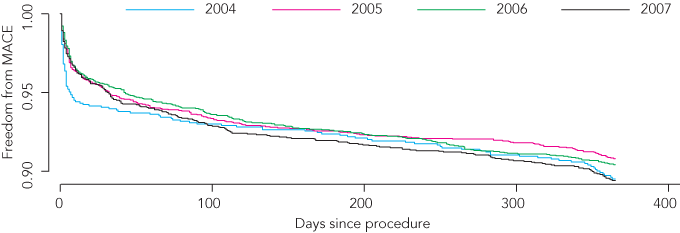
Twelve-month follow-up was completed for 96.5% of procedures. Overall rates of MI (mean, 4.8%), death from any cause (3.8%) and TVR (6.8%) remained stable, but stent thrombosis (1.8%) increased significantly (P = 0.03) (Box 5). Kaplan–Meier estimates of 12-month MACE-free survival were similar for each year studied (P = 0.06) (Box 6). Unadjusted MACE rates at 12 months were lower in patients who received DES compared with BMS, driven mainly by lower TVR rates (Box 7).
After adjustment, multivariate analysis demonstrated that use of DES was the only independent predictor of freedom from MACE at 12 months (P < 0.01), whereas increasing age, diabetes mellitus, renal failure, cardiogenic shock, peripheral arterial disease and multivessel disease were some of the independent predictors of 12-month MACE (all P < 0.01) (Box 8).
Our study represents the largest contemporary Australian multicentre analysis of PCI practice in the DES era. We observed several trends from 2004–2005 to 2007–2008, including increasing patient risk profile and lesion complexity; declining use of DES; an increase in planned clopidogrel therapy of ≥ 12 months after DES; high stable rates of procedural success and low rates of 12-month adverse outcomes; and selective use of DES independently predicting improved outcome at 12 months.
National data show that although overall use of PCI in Australia has increased considerably since its introduction in the 1980s, its growth has slowed in recent years.1 The reasons for this are unknown, but may include reduced need for re-intervention with the advent of DES, and aggressive secondary prevention contributing to reduced cardiovascular event rates. Findings from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, which showed that PCI had no mortality benefit over optimal medical therapy in patients with stable coronary artery disease,20 have been shown to have an impact on the management of stable angina, with catheterisation referral volume decreasing, medication use increasing, and the use of medical therapy rather than revascularisation increasing among patients with coronary disease.21 Our findings of decreasing elective and increasing urgent PCI, especially for MI < 24 hours, support these observations.
There is considerable evidence that PCI in the setting of acute coronary syndromes reduces death and recurrent MI.22 PCI is increasingly used to treat patients with acute MI. In particular, primary PCI is becoming the strategy of choice in most hospitals with cardiac catheterisation laboratory capabilities, and use of primary PCI has grown considerably in recent years due to 24-hour primary PCI services in most centres. According to the National Coronary Angioplasty Register, in Australia in 1999, the main uses of PCI were for stable angina (42%), unstable angina (42%) and acute MI (9%).23 In our registry, acute coronary syndromes accounted for more than 60% of cases (> 20% STEMI). The increase in in-hospital incidence of major bleeding that we found may be related to increased proportions of patients with acute MI and use of glycoprotein IIb/IIIa inhibitors, which are known predictors of increased bleeding risk.24
The high rate of stent use has enabled more complex procedures to be undertaken in more acute situations. In 1995, coronary stents were used in 30% of PCI procedures as bail-out for complications, but, by 2000, they were used in 89% of cases.1 We have shown that overall stent insertion rates were close to 95%, although use of DES declined to 32% in 2007–2008 (in line with the Victorian Department of Human Services recommendation for 30%–40% use).16
Concerns over the safety of DES linked to the risk of late stent thrombosis were first raised in 2004.25 Although subsequent meta-analyses and large observational studies have shown efficacy of DES without major safety concerns, this controversy has influenced clinical decisions, with many registries demonstrating lower use of DES during 2007.3,26 Our data suggest that use of DES was already decreasing before 2006, when the first large study demonstrating safety concerns was presented.6 Patient selection for DES in the MIG registry hospitals remains focused on those at highest risk of restenosis, who should be tolerant of dual antiplatelet therapy. The rate of use of DES in the long term remains uncertain, and will be strongly influenced by the efficacy and safety balance of newer-generation DES.
We have previously reported that in Victorian public hospitals, DES have been used predominantly in patients with risk factors for restenosis (87.7% of PCIs with DES had at least one criterion for high risk of restenosis),4 in accordance with current guidelines. However, many patients deemed at high risk of restenosis did not receive DES, and we hypothesised at the time that greater use of DES in these patients may improve clinical outcomes by reducing restenosis. We have also previously reported that patients who received DES had similar mortality rates to those who received BMS (propensity score-adjusted OR, 0.82 [95% CI, 0.56–1.20]; P = 0.31) and significantly lower TVR rates (propensity score-adjusted OR, 0.66 [95% CI, 0.48–0.90]; P < 0.01) at 12 months.27 In the current study, despite increasing patient risk profile and lesion complexity, highly selective use of DES in 32% of PCI procedures in 2007–2008 achieved comparable low rates of adverse outcomes at 12 months to those seen with higher use of DES (54% of PCI) in 2004–2005. Furthermore, selective use of DES is likely to be a more cost-effective strategy.28
Dual antiplatelet therapy (aspirin and clopidogrel) was almost universally prescribed for at least 12 months after DES in 2007–2008, as per the recommendation of the ACC/AHA guidelines (released in late 2007) for patients at low risk of bleeding.19 We recently reported results showing the clear benefits of longer dual antiplatelet therapy.29 Given that the optimal duration of clopidogrel therapy after DES is not established, the dramatic increase in the proportion of patients prescribed ≥ 12 months of clopidogrel after DES in this study may suggest a trend towards indefinite use of dual antiplatelet therapy in clinical practice.
Our study has some limitations. As not all Victorian public hospitals were represented, our findings may not reflect PCI practice in non-participating hospitals. A registry has inherent limitations and biases that may not be completely adjusted for by modelling. For example, the choice of stent was at the discretion of the operator, and some of the procedural and patient factors precluding the use of DES may not have been captured. Appraisal of low-frequency clinical events such as late thrombosis is limited. The MIG registry has now been linked to the National Death Index to acquire longer-term (> 12 months) mortality rates. Finally, the MIG registry is procedure-based rather than patient-based, so any patients who underwent multiple PCIs during the study period were not accounted for. We are currently performing patient-specific analysis to assess specific cohorts, such as patients returning on multiple occasions with in-stent restenosis.
Interventional cardiology continues to evolve in respect to selection of patients, devices used, and adjunctive drug treatment. Despite increasing risk profiles of patients undergoing PCI, procedural success has remained high and adverse outcomes remain low. These results were achieved with more selective use of DES and longer duration of dual antiplatelet therapy.
1 Trends in baseline characteristics for patients undergoing percutaneous coronary intervention (PCI), by year*
|
Overall |
2004 |
2005 |
2006 |
2007 |
P |
|||||||||
Procedures |
9204 |
1195 |
2665 |
2926 |
2418 |
|
|||||||||
Mean patient age (years) |
64.6 |
64.7 |
64.9 |
64.3 |
64.7 |
0.45 |
|||||||||
Age group (years) |
|
|
|
|
|
|
|||||||||
< 65 |
48.0% |
46.9% |
46.5% |
49.3% |
48.7% |
|
|||||||||
65–80 |
41.1% |
42.1% |
42.2% |
40.3% |
40.4% |
|
|||||||||
> 80 |
10.9% |
11.0% |
11.3% |
10.4% |
10.9% |
|
|||||||||
Male |
74.7% |
72.9% |
73.3% |
75.1% |
76.5% |
< 0.01 |
|||||||||
Comorbidities |
|
|
|
|
|
|
|||||||||
Diabetes mellitus |
24.0% |
23.6% |
22.5% |
24.5% |
25.3% |
0.05 |
|||||||||
Hypertension |
64.3% |
60.7% |
62.7% |
65.0% |
67.0% |
< 0.01 |
|||||||||
Hyperlipidaemia |
71.3% |
64.9% |
73.1% |
72.1% |
71.4% |
0.02 |
|||||||||
Current smoker |
22.5% |
22.0% |
21.8% |
23.0% |
22.9% |
0.32 |
|||||||||
Peripheral arterial disease |
7.0% |
5.7% |
6.5% |
6.8% |
8.2% |
< 0.01 |
|||||||||
Stroke |
5.9% |
4.6% |
5.4% |
6.2% |
6.6% |
< 0.01 |
|||||||||
Moderate/severe renal disease |
4.1% |
5.3% |
4.0% |
0.3% |
4.8% |
0.73 |
|||||||||
Cardiac history |
|
|
|
|
|
|
|||||||||
History of myocardial infarction |
30.4% |
31.8% |
27.3% |
32.1% |
31.1% |
0.14 |
|||||||||
History of heart failure |
3.8% |
3.7% |
3.5% |
4.0% |
4.2% |
0.24 |
|||||||||
Prior PCI |
24.4% |
24.1% |
24.1% |
24.1% |
25.3% |
0.37 |
|||||||||
Prior coronary artery bypass graft |
9.4% |
7.5% |
9.7% |
9.8% |
9.6% |
0.13 |
|||||||||
* Each year is from 1 April to 31 March the following year. |
|||||||||||||||
2 Trends in percutaneous coronary intervention procedural characteristics, by year*
|
Overall |
2004 |
2005 |
2006 |
2007 |
P |
|||||||||
Procedures |
9204 |
1195 |
2665 |
2926 |
2418 |
|
|||||||||
Procedure type |
|
|
|
|
|
|
|||||||||
Elective procedure |
44.8% |
56.9% |
52.0% |
44.2% |
35.5% |
< 0.01 |
|||||||||
Urgent procedure |
53.3% |
40.7% |
45.8% |
56.9% |
63.3% |
< 0.01 |
|||||||||
Rescue procedure |
1.9% |
2.4% |
2.2% |
1.9% |
1.2% |
< 0.01 |
|||||||||
Myocardial infarction (MI), < 24 hours |
23.0% |
17.6% |
20.4% |
24.2% |
27.2% |
< 0.01 |
|||||||||
MI, 1–7 days |
23.2% |
16.4% |
20.0% |
25.3% |
27.7% |
< 0.01 |
|||||||||
Acute coronary syndrome |
61.5% |
63.4% |
60.3% |
59.4% |
64.4% |
0.22 |
|||||||||
ST-elevation MI |
24.4% |
19.6% |
20.5% |
26.5% |
28.4% |
< 0.01 |
|||||||||
Non-ST-elevation MI |
23.7% |
11.0% |
28.1% |
31.5% |
26.6% |
< 0.01 |
|||||||||
Shock |
2.3% |
2.3% |
2.0% |
2.3% |
2.8% |
0.13 |
|||||||||
Heart failure at presentation |
5.0% |
6.0% |
6.0% |
4.9% |
5.6% |
0.35 |
|||||||||
Cardiac anatomy and function |
|
|
|
|
|
|
|||||||||
Ejection fraction < 40% |
10.9% |
10.3% |
11.3% |
10.1% |
11.5% |
0.06 |
|||||||||
Multivessel disease |
59.0% |
56.3% |
59.1% |
57.8% |
61.1% |
0.05 |
|||||||||
Mean number of lesions treated |
1.21 |
1.28 |
1.22 |
1.21 |
1.18 |
< 0.01 |
|||||||||
Glycoprotein IIb/IIIa inhibitor |
28.1% |
27.7% |
25.2% |
28.6% |
30.8% |
< 0.01 |
|||||||||
Vessel treated |
|
|
|
|
|
|
|||||||||
Left main coronary artery |
0.9% |
0.7% |
0.8% |
0.7% |
1.2% |
0.07 |
|||||||||
Left anterior descending artery |
33.1% |
32.4% |
31.3% |
34.1% |
34.2% |
0.03 |
|||||||||
Left circumflex artery |
13.9% |
15.4% |
13.6% |
13.4% |
14.0% |
0.33 |
|||||||||
Right coronary artery |
31.5% |
31.1% |
32.3% |
31.8% |
30.5% |
0.40 |
|||||||||
Bypass graft |
3.0% |
1.8% |
3.3% |
3.1% |
3.1% |
0.10 |
|||||||||
Lesion characteristics |
|
|
|
|
|
|
|||||||||
ACC/AHA type B2 or C |
48.4% |
45.0% |
48.8% |
51.3% |
46.1% |
0.68 |
|||||||||
In-stent restenosis |
5.8% |
5.1% |
5.6% |
5.7% |
6.8% |
0.02 |
|||||||||
Bifurcation |
9.1% |
6.6% |
7.3% |
8.4% |
13.6% |
< 0.01 |
|||||||||
Chronic total occlusion |
3.2% |
0.9% |
3.2% |
4.2% |
3.3% |
< 0.01 |
|||||||||
Ostial |
5.3% |
4.4% |
4.2% |
5.1% |
7.4% |
< 0.01 |
|||||||||
Mean maximum stent/balloon size (mm) |
3.03 |
2.93 |
2.98 |
3.04 |
3.12 |
< 0.01 |
|||||||||
Mean stent length (mm) |
18.54 |
18.26 |
18.60 |
18.56 |
18.62 |
0.62 |
|||||||||
Devices used |
|
|
|
|
|
|
|||||||||
Stents, any |
94.6% |
94.9% |
94.8% |
94.8% |
93.8% |
< 0.01 |
|||||||||
Bare-metal stent (BMS) |
53.3% |
46.5% |
47.9% |
52.3% |
63.8% |
< 0.01 |
|||||||||
Drug-eluting stent (DES) |
44.7% |
53.9% |
50.5% |
46.1% |
32.0% |
< 0.01 |
|||||||||
Mixed BMS and DES |
3.4% |
5.4% |
3.6% |
3.6% |
2.1% |
< 0.01 |
|||||||||
Balloon angioplasty alone |
5.4% |
5.1% |
5.2% |
5.2% |
6.2% |
|
|||||||||
Procedural success |
95.9% |
95.7% |
95.9% |
95.6% |
96.3% |
0.37 |
|||||||||
ACC/AHA = American College of Cardiology/American Heart Association. * Each year is from 1 April to 31 March the following year. |
|||||||||||||||
3 Odds ratios* for likelihood of receiving a drug-eluting stent
 | |||||||||||||||
|
ACS = acute coronary syndrome. CABG = coronary artery bypass graft. CHF = congestive heart failure. CTO = chronic total occlusion. ISR = in-stent restenosis. LAD = left anterior descending. LMCA = left main coronary artery. PAD = peripheral arterial disease. PCI = percutaneous coronary intervention. RCA = right coronary artery. STEMI = ST-elevation myocardial infarction. * Bars indicate 95% CIs. † 2004–2005 is reference group. Each year is from 1 April to 31 March the following year. | |||||||||||||||
4 Planned duration of clopidogrel therapy, by stent type and year*
Months |
Overall |
2004 |
2005 |
2006 |
2007 |
||||||||||
1† |
24.7% |
18.3% |
22.4% |
21.6% |
33.9% |
||||||||||
BMS |
46.1% |
41.5% |
46.8% |
41.2% |
51.6% |
||||||||||
DES |
0.7% |
0.6% |
0.7% |
0.5% |
0.9% |
||||||||||
3† |
6.3% |
9.5% |
8.0% |
5.2% |
4.4% |
||||||||||
BMS |
7.9% |
6.3% |
9.6% |
8.7% |
6.5% |
||||||||||
DES |
4.4% |
11.6% |
6.6% |
1.3% |
0.3% |
||||||||||
6† |
15.6% |
28.8% |
25.0% |
12.0% |
3.2% |
||||||||||
BMS |
12.3% |
24.4% |
19.8% |
10.3% |
4.4% |
||||||||||
DES |
19.7% |
33.0% |
29.7% |
14.3% |
0.8% |
||||||||||
≥ 12† |
53.4% |
43.4% |
44.6% |
61.1% |
58.5% |
||||||||||
BMS |
29.8% |
27.8% |
23.8% |
39.8% |
37.6% |
||||||||||
DES |
57.2% |
54.7% |
63.0% |
73.8% |
98.0% |
||||||||||
BMS = bare-metal stents. DES = drug-eluting stents. * Each year is from 1 April to 31 March the following year. † P for trend < 0.01. |
|||||||||||||||
5 Clinical outcomes, by year*
|
Overall |
2004 |
2005 |
2006 |
2007 |
P (trend) |
|||||||||
Mean length of stay, days |
3.6 |
3.2 |
3.2 |
3.8 |
3.8 |
< 0.001 |
|||||||||
In-hospital outcomes (n) |
9204 |
1195 |
2665 |
2926 |
2418 |
|
|||||||||
Death |
1.5% |
1.3% |
1.5% |
1.5% |
1.5% |
0.64 |
|||||||||
Myocardial infarction |
1.5% |
1.8% |
1.1% |
1.2% |
1.6% |
0.15 |
|||||||||
Major bleeding |
1.9% |
1.0% |
1.5% |
2.4% |
2.3% |
0.001 |
|||||||||
Stroke |
0.2% |
0.6% |
0.2% |
0.1% |
0.2% |
0.03 |
|||||||||
Unplanned CABG |
0.7% |
0.6% |
0.9% |
0.6% |
0.6% |
0.51 |
|||||||||
30-day outcomes (n) |
9204 |
1195 |
2665 |
2926 |
2418 |
|
|||||||||
Death |
1.8% |
1.4% |
2.0% |
1.7% |
2.0% |
0.5 |
|||||||||
Myocardial infarction |
2.2% |
3.1% |
1.8% |
2.2% |
2.5% |
0.81 |
|||||||||
Target vessel revascularisation |
2.1% |
2.5% |
2.0% |
2.2% |
2.0% |
0.49 |
|||||||||
MACE |
5.3% |
6.5% |
5.1% |
1.9% |
5.3% |
0.31 |
|||||||||
Stent thrombosis |
0.6% |
0.3% |
0.4% |
0.8% |
0.8% |
0.01 |
|||||||||
12-month outcomes (n) |
8885 |
1166 |
2631 |
2775 |
2313 |
|
|||||||||
Death |
3.8% |
2.7% |
4.0% |
4.0% |
4.0% |
0.41 |
|||||||||
Myocardial infarction |
4.8% |
5.1% |
3.9% |
4.9% |
5.4% |
0.17 |
|||||||||
Target vessel revascularisation |
6.8% |
7.5% |
6.5% |
6.8% |
8.2% |
0.18 |
|||||||||
MACE |
12.6% |
13.3% |
12.3% |
12.7% |
14.7% |
0.09 |
|||||||||
Stent thrombosis, > 30 days |
1.0% |
0.8% |
0.9% |
1.1% |
1.1% |
0.32 |
|||||||||
Stent thrombosis, total |
1.8% |
1.5% |
1.3% |
2.3% |
2.2% |
0.03 |
|||||||||
CABG = coronary artery bypass graft. MACE = major adverse cardiac events (composite of death, myocardial infarction and target vessel revascularisation). * Each year is from 1 April to 31 March the following year.
6 Estimates of freedom from major adverse cardiac events (MACE),* by year†
 | |||||||||||||||
|
* Composite of death, myocardial infarction and target vessel revascularisation. † Each year is from 1 April to 31 March the following year. P = 0.06. | |||||||||||||||
7 Clinical outcomes at 12 months, by stent type and year*
|
Drug-eluting stents |
Bare-metal stents |
|||||||||||||
|
2004 |
2005 |
2006 |
2007 |
P† |
2004 |
2005 |
2006 |
2007 |
P† |
|||||
Death |
1.4% |
3.7% |
3.0% |
3.0% |
0.05 |
3.8% |
4.0% |
4.6% |
4.3% |
0.88 |
|||||
MI |
5.6% |
3.9% |
4.5% |
6.6% |
0.50 |
4.4% |
4.2% |
4.6% |
4.3% |
0.76 |
|||||
TVR |
7.0% |
4.7% |
4.9% |
5.2% |
0.10 |
6.9% |
6.7% |
6.7% |
8.0% |
0.85 |
|||||
MACE |
12.4% |
10.3% |
10.1% |
12.3% |
0.16 |
12.8% |
12.4% |
12.6% |
14.2% |
0.93 |
|||||
MI = myocardial infarction. TVR = target vessel revascularisation. MACE = major adverse cardiac events (composite of death, MI and TVR). * Each year is from 1 April to 31 March the following year. † For trend. |
|||||||||||||||
8 Independent predictors of 12-month major adverse cardiac events
Variable |
Odds ratio (95% CI) |
||||||||||||||
Drug-eluting stent |
0.68 (0.56–0.81)* |
||||||||||||||
Age (per year increase) |
1.01 (1.00–1.02)* |
||||||||||||||
Glycoprotein IIb/IIIa use |
1.32 (1.11–1.58)* |
||||||||||||||
Diabetes mellitus |
1.30 (1.10–1.54)* |
||||||||||||||
Left anterior descending artery |
1.34 (1.11–1.61)* |
||||||||||||||
Cerebrovascular disease |
1.40 (1.08–1.82)† |
||||||||||||||
Peripheral arterial disease |
1.48 (1.15–1.91)* |
||||||||||||||
Multivessel disease |
1.53 (1.29–1.81)* |
||||||||||||||
Renal failure |
2.26 (1.70–3.01)* |
||||||||||||||
Cardiogenic shock |
4.36 (3.01–6.33)* |
||||||||||||||
* P < 0.01. † P = 0.01. |
|||||||||||||||
Received 3 May 2010, accepted 22 February 2011
- Bryan P Yan1,2
- Andrew E Ajani2,3
- David J Clark4
- Stephen J Duffy5
- Nick Andrianopoulos2,6
- Angela L Brennan2
- Philippa Loane2
- Christopher M Reid2
- 1 Chinese University of Hong Kong, Hong Kong.
- 2 Monash Centre of Cardiovascular Research & Education in Therapeutics, Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 3 Royal Melbourne Hospital, Melbourne, VIC.
- 4 Austin Hospital, Melbourne, VIC.
- 5 Alfred Hospital, Melbourne, VIC.
- 6 Centre for Research Excellence in Patient Safety, Monash University, Melbourne, VIC.
MIG Investigators: Alfred Hospital: S Duffy, J Shaw, A Walton, R Johnston, A Dart, A Broughton, J Federman, C Keighley, C Hengel, K Peter, W Chan, L Lefkovits, A Kularatne, D Stub, S McKenzie; Austin Hospital: D Clark, O Farouque, M Horrigan, J Johns, L Oliver, J Brennan, R Chan, G Proimos, T Dortimer, A Tonkin, K Charter, L Brown, A Sahar, M Freeman, M Mok, A Al-Fiadh, M Wong, T Lancefield; Box Hill Hospital: G New, L Roberts, M Rowe, G Proimos, Y Cheong, C Goods, D Fernando, J Coller, M Moore, J Beale; Frankston Hospital: R Lew, G Szto, R Teperman, R Huq, C Shaw; Geelong Hospital: A Black, M Sebastian, T Yip, C Hiew, J Dyson, T Du Plessis; Monash University: H Krum, C Reid, N Andrianopoulos, A Brennan, P Loane, L Curran, A Finlay, D Dinh, C Steer; Northern Hospital: W van Gaal, P Barlis, E Buckley; Royal Melbourne Hospital: A Ajani, R Warren, D Eccleston, J Lefkovits, B Yan, R Iyer, W Wilson, R Gurvitch, I Harries, M Watts, A Sharifi, S Ruane; Western Hospital: Y-L Lim, D Eccleston, A Walton.
The MIG acknowledges unrestricted educational grant funding from Abbot Vascular, AstraZeneca, Biotronik, Boston Scientific, Bristol-Myers Squibb, CSL, Johnson & Johnson, Medtronic, Pfizer, Schering-Plough, Sanofi-Aventis and Servier. These companies do not have access to the data nor the right to review manuscripts before publication. Stephen Duffy’s work is funded by a National Health and Medical Research Council Program Grant.
None relevant to this article declared (ICMJE disclosure forms completed).
- 1. Davies J. Coronary revascularisation in Australia, 2000. AIHW bulletin no. 7. Canberra: Australian Institute of Health and Welfare, 2003. (AIHW Cat. No. AUS 35.)
- 2. Hilliard AA, From AM, Lennon RJ, et al. Percutaneous revascularization for stable coronary artery disease temporal trends and impact of drug-eluting stents. JACC Cardiovasc Interv 2010; 3: 172-179.
- 3. Austin D, Oldroyd KG, Holmes DR Jr, et al; APPROACH Investigators; Belgian Working Group on Invasive Cardiology; Mayo Clinic PCI Registry; Scottish Coronary Revascularisation Registry. Drug-eluting stents: a study of international practice. Am Heart J 2009; 158: 576-584.
- 4. Yan BP, Ajani AE, Duffy SJ, et al; Melbourne Interventional Group investigators. Use of drug-eluting stents in Victorian public hospitals. Med J Aust 2006; 185: 363-367. <MJA full text>
- 5. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293: 2126-2130.
- 6. Holmes DR Jr, Kereiakes DJ, Laskey WK, et al. Thrombosis and drug-eluting stents: an objective appraisal. J Am Coll Cardiol 2007; 50: 109-118.
- 7. Lagerqvist B, James SK, Stenestrand U, et al; SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med 2007; 356: 1009-1019.
- 8. Ellis SG, Stone GW, Cox DA, et al; TAXUS IV Investigators. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc Interv 2009; 2: 1248-1259.
- 9. Caixeta A, Leon MB, Lansky AJ, et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol 2009; 54: 894-902.
- 10. Weerasinghe DP, Yusuf F, Parr NJ. Trends in percutaneous coronary interventions in New South Wales, Australia. Int J Environ Res Public Health 2009; 6: 232-245.
- 11. Hobbs MS, McCaul KA, Knuiman MW, et al. Trends in coronary artery revascularisation procedures in Western Australia, 1980–2001. Heart 2004; 90: 1036-1041.
- 12. Ajani AE, Szto G, Duffy SJ, et al; Melbourne Interventional Group investigators. The foundation and launch of the Melbourne Interventional Group: a collaborative interventional cardiology project. Heart Lung Circ 2006; 15: 44-47.
- 13. McNeil JJ, Evans SM, Johnson NP, Cameron PA. Clinical-quality registries: their role in quality improvement [editorial]. Med J Aust 2010; 192: 244-245. <MJA full text>
- 14. Skillington P. A National Cardiac Surgery Database: current achievements and future challenges. Heart Lung Circ 2001; 10 (1 Suppl): S2-S4.
- 15. Graves SE, Davidson D, Ingerson L, et al. The Australian Orthopaedic Association National Joint Replacement Registry. Med J Aust 2004; 180 (5 Suppl): S31-S34. <MJA full text>
- 16. Fennessy P. Implementing a new technology/clinical practice: the DHS view. Case study: coronary artery drug-eluting stents. Melbourne: Department of Human Services. http://www.health.vic. gov.au/newtech/documents/fennessy.pdf (accessed Oct 2010).
- 17. Chan W, Clark DJ, Ajani AE, et al. Progress towards a National Cardiac Procedure Database — development of the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and Melbourne Interventional Group (MIG) registries. Heart Lung Circ 2011; 20: 10-18.
- 18. Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol 2007; 49: 734-739.
- 19. King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, writing on behalf of the 2005 Writing Committee. Circulation 2008; 117: 261-295.
- 20. Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503-1516.
- 21. Atwater BD, Oujiri J, Wolff MR. The immediate impact of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial on the management of stable angina. Clin Cardiol 2009; 32 (8): E1-E3.
- 22. Fox KA, Clayton TC, Damman P, et al; FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55: 2435-2445. Epub 2010 Mar 30.
- 23. Davies J, Senes S. Coronary angioplasty in Australia 1999. Cardiovascular disease series no. 19. Canberra: Australian Institute of Health and Welfare and National Heart Foundation of Australia, 2002. (AIHW Cat. No. CVD 19.)
- 24. Mehta SK, Frutkin AD, Lindsey JB, et al; National Cardiovascular Data Registry. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv 2009; 2: 222-229.
- 25. McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004; 364: 1519-1521.
- 26. Roe MT, Chen AY, Cannon CP, et al; CRUSADE and ACTION–GWTG Registry Participants. Temporal changes in the use of drug-eluting stents for patients with non-ST-segment-elevation myocardial infarction undergoing percutaneous coronary intervention from 2006 to 2008: results from the Can Rapid risk stratification of Unstable angina patients supress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) and Acute Coronary Treatment and Intervention Outcomes Network–Get With The Guidelines (ACTION–GWTG) Registries. Circ Cardiovasc Qual Outcomes 2009; 2: 414-420.
- 27. Ajani AE, Reid CM, Duffy SJ, et al. Outcomes after percutaneous coronary intervention in contemporary Australian practice: insights from a large multicentre registry. Med J Aust 2008; 189: 423-428. <MJA full text>
- 28. Goeree R, Bowen JM, Blackhouse G, et al. Economic evaluation of drug-eluting stents compared to bare metal stents using a large prospective study in Ontario. Int J Technol Assess Health Care 2009; 25: 196-207.
- 29. Butler MJ, Eccleston D, Clark DJ, et al; Melbourne Interventional Group. The effect of intended duration of clopidogrel use on early and late mortality and major adverse cardiac events in patients with drug-eluting stents. Am Heart J 2009; 157: 899-907.





Abstract
Objective: To evaluate percutaneous coronary intervention (PCI) practice trends and 12-month outcomes in Australia in the era of drug-eluting stents (DES).
Design, setting and patients: Prospective study of consecutive patients undergoing 9204 PCIs between 1 April 2004 and 31 March 2008 at seven Victorian public hospitals.
Main outcome measures: Temporal trends in baseline characteristics and in-hospital and 12-month clinical outcomes including death, myocardial infarction (MI), target vessel revascularisation (TVR) and composite major adverse cardiac events (MACE), from year to year.
Results: Between 2004–2005 and 2007–2008, the mean age of patients undergoing PCI was stable (65 ± 12 years), and comorbidities such as hypertension, hyperlipidaemia, peripheral arterial disease and stroke increased (P < 0.05). There were fewer elective and more urgent PCIs, especially for MI < 24 hours (17.6% in 2004–2005 to 27.2% in 2007–2008, P < 0.01). Overall stent use remained high (mean, 94.6%), but use of DES declined steadily (53.9% in 2004–2005 to 32.0% in 2007–2008, P < 0.01), despite increases in complex lesions. Planned clopidogrel therapy of ≥ 12 months after insertion of DES increased from 54.7% in 2004–2005 to 98.0% in 2007–2008 (P < 0.01). The overall procedural success rate was high (mean, 95.9%), and 12-month rates of mortality (3.8%), MI (4.8%), TVR (6.8%) and stent thrombosis (1.8%) remained low. Selective use of DES was an independent predictor of freedom from MACE at 12 months (odds ratio, 0.68; 95% CI, 0.56–0.81).
Conclusions: Use of DES declined steadily from 2004–2005 to 2007–2008, despite increasing patient risk profile and lesion complexity. Procedural success remained high and 12-month adverse outcomes remained low, with increasing use of prolonged dual antiplatelet therapy.