Stent implantation has significantly improved the short-term and long-term outcomes of patients undergoing percutaneous coronary interventions (PCIs) for obstructive coronary artery disease compared with balloon angioplasty alone.1 However, in-stent restenosis may lead to recurrent ischaemia and repeat intervention at rates approaching 30% in high-risk patient subgroups, including those with diabetes, long lesions and small vessels.2-4 Recently, drug-eluting stents (DESs) that are impregnated with anti-proliferative agents have emerged as an effective strategy in preventing restenosis.5,6 In two large randomised controlled trials evaluating stents eluting paclitaxel and sirolimus, there was about a 50% reduction in the rate of target vessel failure (defined by death, myocardial infarction, or patients having undergone target vessel revascularisation) in patients receiving DESs compared with conventional bare-metal stents (BMSs).7,8
Our data were part of those collected for the Melbourne Interventional Group registry. This registry is a voluntary, collaborative venture by interventional cardiologists practising at these seven hospitals, designed to record data pertaining to PCI and to perform long-term follow-up. Demographic, clinical and procedural characteristics of consecutive patients undergoing PCI are prospectively recorded on a standard case report form with standardised definitions for all fields.9 The registry is coordinated by the Centre of Clinical Research Excellence in Therapeutics, a research body within the Department of Epidemiology and Preventive Medicine at Monash University, Melbourne. Case record forms for the collection of registry data have been developed using Teleform, version 9 (Cardiff, Vista, Calif, USA). Completed forms are faxed to the data centre, verified on receipt, and electronically uploaded into the central database. A query system has been developed to identify missing data, data inconsistencies and out-of-range values. The database is built on a Microsoft SQL Server platform (Microsoft Corporation, Redmond, Wash, USA) with a Microsoft Access (Microsoft Corporation, Redmond, Wash, USA) user interface.
The study protocol was approved by the ethics committee in each participating hospital. “Opt-out” informed consent was obtained in all patients, as previously described.9
The interventional strategy and stent selection was left to the discretion of the operator in all procedures. Total stent length was used as a surrogate measure for target lesion length, and stent diameter for target vessel diameter. Periprocedural glycoprotein IIb/IIIa inhibitors were used according to the operator’s decision. Oral antiplatelet therapy followed current internationally accepted guidelines, which recommend combination of aspirin and clopidogrel for a minimum of 4 weeks for BMSs and for 6–12 months for DESs.10
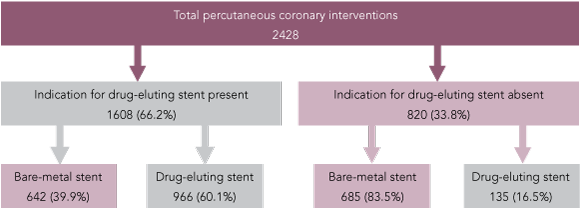
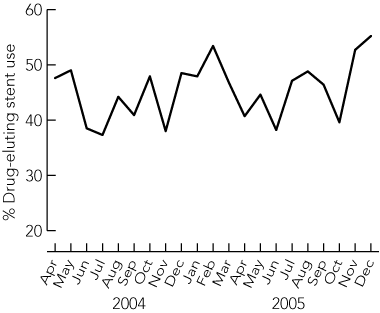
There were 2428 PCI procedures, with stent implantation in 2976 coronary artery lesions during the study period. Of the 2428 PCIs, 1101 (45.3%) involved insertion of at least one DES, and BMSs were inserted in the remaining 1327 (54.7%). The proportion of DES use was stable over the study period (Box 1). The rates of PCI involving BMSs and DESs according to whether Department of Human Services criteria for DESs were present are shown (Box 2). In 87.7% of PCIs in which DESs were implanted (966/1101), there was at least one Department of Human Services criterion for DES use. However, of the total 2428 PCIs, 1608 (66.2%) were eligible for a DES according to Department of Human Services criteria, and in 642 (39.9%) of these procedures in patients at high-risk of restenosis, only BMSs were used.
Patients treated with DESs had more diabetes (32.5% v 16.5%; P < 0.01), previous myocardial infarction (30.8% v 27.4%; P = 0.04) and previous coronary artery bypass graft (10.3% v 6.3%; P < 0.01) than those in whom only BMSs were used (Box 3). A greater proportion of left-anterior-descending artery and left-main-stem lesions were treated with DESs compared with BMSs (37.7% v 27.7%; P < 0.01 and 1.1% v 0.5%; P < 0.01, respectively). More lesions treated with DESs were complex (American College of Cardiology/American Heart Association, type B2/C lesions) than those treated with BMSs (55% v 41.3%; P < 0.01). More DESs than BMSs were implanted in small vessels (≤ 2.5 mm stents; 45.2% v 17%; P < 0.01), long lesions requiring 20 mm or more in length of stent (46.9% v 17.3%; P < 0.01), chronic total occlusions (1.4% v 0.8%; P < 0.01), bifurcation lesions (8.6% v 5.6%; P = 0.02) and in-stent restenotic lesions (7.9% v 2%; P < 0.01) (Box 4). Conversely, in patients who presented with ST-elevation myocardial infarction (STEMI) and cardiogenic shock, more were treated with BMSs than DESs (23.3% v 17.5%; P = 0.01 and 1.4% v 0.9%; P = 0.22, respectively). There was no significant difference in stent preference in patients presenting with non-ST-elevation acute coronary syndromes.
The percentages of PCIs in which DESs were used in accordance with Department of Human Services criteria is shown in Box 5. DES use ranged from a high of 75.6% for in-stent restenosis down to 52.0% for chronic total occlusions. The likelihood of receiving a DES increased with the number of criteria satisfied (Box 6), from an RR of 1.34 (95% CI, 1.14–1.58) with one criterion present to a RR of 10.41 (95% CI, 6.13–17.5) when three or more criteria were present.
The uptake of DESs by the interventional cardiology community is not uniform. In the United States, 17 266 PCIs were performed in Veteran Health Administration medical centres from 2002 to 2004, with DES use reported in 52% of cases.11 On the other hand, a German registry of 3579 interventions at 102 centres reported less than 10% DES use between April 2002 and December 2003.12 In Australia, DES use in public hospitals varies considerably between states. There are very high rates of DES use (about 90%) in Western Australia, compared with about 60% in South Australia and 50% in New South Wales, where rates in some hospitals were less than 10% (J Rankin, Interventional Cardiologist, Royal Perth Hospital, Perth, WA; D P Chew, Interventional Cardiologist, Flinders Medical Centre, Bedford Park, SA; and D M Muller, Interventional Cardiologist, Director of Cardiac Catheterisation Laboratory, St Vincent’s Hospital, Darlinghurst, NSW; personal communications, March 2006). A recent preliminary report found DES use in Victorian private hospitals exceeded 94% — about twice that of Victorian public hospitals.13
The rationale for selective use of DESs is twofold. Firstly, the greatest clinical benefit of DESs is expected for patients at the highest risk of restenosis. A number of clinical and angiographic features are known to increase the risk of restenosis after bare-metal stenting.14-21 Lesion-related factors are described above. The major patient-related factor is diabetes mellitus, which doubles the risk of in-stent restenosis.20,21 DESs have been shown to be safe and effective in each of these subgroups.14,16-18,22-25 A recent study showed the strategy of selective DES use in patients with high-risk features (including diabetes, left ventricular ejection fraction < 35%, lesions in the left anterior descending artery and left main stem artery, saphenous vein grafts, chronic total occlusions, ostial or bifurcation lesions) was associated with a significant decrease in major adverse cardiac events, defined as a composite of death, myocardial infarction and target vessel revascularisation (hazard ratio [HR], 0.45; 95% CI, 0.29–0.72), whereas no difference was observed in patients without high-risk features (HR, 0.95; 95% CI, 0.40–2.28).26
Secondly, unrestricted DES use is not economically viable under the public health system. A recent cost-effectiveness analysis in the US suggested the sirolimus-eluting stent would be a cost effective treatment strategy when the rate of restenosis exceeds 18.5%.27 However, not all patients undergoing PCI are at high risk of restenosis. A recent study of 5239 patients undergoing PCI identified factors (eg, native vessels, de novo lesions, reference diameter > 3.5 mm, lesion length < 5 mm, absence of diabetes and non-ostial lesions) which predicted a low (4%–10%) risk of repeat revascularisation at 9 months.28 Marginal improvement in outcomes from DES use in these low-risk patients is unlikely to be cost-effective, thus providing the economic basis for current Victorian guidelines. A recent Australian study showed that limiting DES use to patients at the highest risk of restenosis might improve the cost-effectiveness of DESs in an Australian model based on randomised trial results.29
A significant number of high-risk patients in our study did not receive DESs. There are a number of possible explanations for why patients who had an indication for a DES received a BMS. First, implanting DESs in tortuous and calcified vessels is more difficult than implanting newer generation low-profile BMSs. The operator may choose to use a more deliverable BMS instead of a DES in the event of failure to deliver a DES. Second, prolonged dual antiplatelet therapy including clopidogrel, which is mandatory after DES implantation, may be undesirable in patients awaiting non-cardiac surgery, at high risk of bleeding or unable to comply with prolonged dual antiplatelet therapy. Third, patients with significant comorbidities or poor prognosis may be excluded. Fourth, acute STEMI was initially considered a relative contraindication for DES use by some operators, resulting in more patients with STEMI receiving BMSs despite having high-risk features, such as diabetes. This stemmed from the lack of randomised trial data and the potential risk of stent thrombosis in the local thrombotic environment of the infarction-related lesion. Recent studies have found DESs to be safe in patients with STEMI.30 Finally, DESs were only funded for 30%–40% of PCIs, and 66.2% of PCIs in this study involved patients with at least one criterion for receiving DESs. Therefore, operators could not use DESs in many appropriate patients without markedly exceeding the allocated budget.
DESs were implanted during some PCIs (16.5%) without an indication (Box 2). In-stent restenosis in the left main and left anterior descending arteries are associated with worse clinical outcome,31-34 and may explain why DESs were more often implanted in these vessels even though target vessel type was not one of the criteria for DES use in Victorian Department of Human Services guidelines.
2 Stent use according to whether percutaneous coronary interventions met Victorian Department of Human Services criteria for implanting drug-eluting stents
3 Baseline characteristics of patients undergoing percutaneous coronary intervention (PCI)
Moderate to severe renal dysfunction (creatinine > 0.20 mmol/L) |
|||||||||||||||
4 Characteristics of percutaneous coronary intervention procedures
ACC/AHA = American College of Cardiology/American Heart Association. |
|||||||||||||||
- Bryan P Yan1
- Andrew E Ajani1,2
- Stephen J Duffy3
- Gishel New2,4
- Mark Horrigan5
- Gregory Szto6
- Antony Walton3,7
- David Eccleston1,7
- Jeffery Lefkovits1
- Alexander Black8
- Martin Sebastian8
- Angela L Brennan2,3
- Christopher M Reid2
- David J Clark5
- on behalf of the Melbourne Interventional Group (MIG) investigators
- 1 Royal Melbourne Hospital, Melbourne, VIC.
- 2 Monash University, Melbourne, VIC.
- 3 Alfred Hospital Heart Centre, Melbourne, VIC.
- 4 Box Hill Hospital, Melbourne, VIC.
- 5 Austin Hospital, Melbourne, VIC.
- 6 Frankston Hospital, Peninsula Health, Frankston, VIC.
- 7 Western Hospital, Melbourne, VIC.
- 8 Geelong Hospital, Geelong, VIC.
The Melbourne Interventional Group acknowledges funding from Astra-Zeneca, Biotronik, Boston Scientific, Guidant, Johnson & Johnson, Pfizer, Sanofi-Aventis, Schering-Plough, Servier, and Terumo. These companies do not have access to the data, and do not have the right to review articles before publication. Dr Duffy is supported by a National Health and Medical Research Council Career Development Award. We are grateful for editorial support from Mardi Malone.
Melbourne Interventional Group (MIG) investigators: The following investigators and institutions participated in the Melbourne Interventional Group registry: Alfred Hospital: S Duffy, J Shaw, A Walton, C Farrington, R Gunaratne, A Broughton, J Federman, C Keighley, A Dart. Austin Hospital: D Clark, J Johns, M Horrigan, O Farouque, L Oliver, J Brennan, R Chan, G Proimos, T Dortimer, B Chan, A Tonkin, L Brown, N Campbell, A Sahar, K Charter. Box Hill Hospital: G New, L Roberts, H Liew, M Rowe, G Proimos, N Cheong, C Goods. Frankston Hospital: R Lew, G Szto, R Templin. Geelong Hospital: A Black, M Sebastian, T Yip, L Ponnuthrai, M Rahmen, J Dyson, T Duplessis. Monash University: H Krum, C Reid, A Brennan, A Meehan, P Loane, L Curran and F Groen. Peninsula Private Hospital: G Szto, V O'Shea. Royal Melbourne Hospital: A Ajani, R Warren, D Eccleston, J Lefkovits, B Yan, P Roy, S Shetty. Western Hospital: Y-L Lim, D Eccleston, A Walton.
None identified.
- 1. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994; 331: 496-501.
- 2. Akiyama T, Moussa I, Reimers B, et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol 1998; 32: 1610-1618.
- 3. Kobayashi Y, De Gregorio J, Kobayashi N, et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol 1999; 34: 651-659.
- 4. Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol 1998; 32: 584-589.
- 5. Hong MK, Mintz GS, Lee CW, et al; Asian Paclitaxel-Eluting Stent Clinical Trial. Paclitaxel coating reduces in-stent intimal hyperplasia in human coronary arteries: a serial volumetric intravascular ultrasound analysis from the Asian Paclitaxel-Eluting Stent Clinical Trial (ASPECT). Circulation 2003; 107: 517-520.
- 6. Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation 2001; 104: 2007-2011.
- 7. Moses JW, Leon MB, Popma JJ, et al; SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349: 1315-1323.
- 8. Stone GW, Ellis SG, Cox DA, et al; TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004; 350: 221-231.
- 9. Ajani AE, Szto G, Duffy SJ, et al, on behalf of the MIG (Melbourne Interventional Group) investigators. The foundation and launch of the Melbourne Interventional Group: a collaborative interventional cardiology project. Heart Lung Circ 2006; 15: 44-47.
- 10. Silber S, Albertsson P, Aviles FF, et al; Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Guidelines for percutaneous coronary interventions. Eur Heart J 2005; 26: 804-847.
- 11. Maynard C, Lowy E, Wagner T, Sales AE. Utilization of drug-eluting stents in the Veterans Health Administration. Am J Cardiol 2005; 96: 218-220.
- 12. Zahn R, Hamm CW, Zeymer U, et al; Deutsche Cyper-Register. [Safety and current indications during “real life” use of sirolimus-eluting coronary stents in Germany. Results from the prospective multicenter German Cypher Registry] [German]. Herz 2004; 29: 181-186.
- 13. Szto G, Eccleston D, Clark D, et al. Does restricted use of drug-eluting stents affect long-term interventional outcomes? [abstract]. Heart Lung Circ 2005; 14(Suppl): S133.
- 14. Ardissino D, Cavallini C, Bramucci E, et al; SES-SMART Investigators. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA 2004; 292: 2727-2734.
- 15. Kobayashi Y, De Gregorio J, Kobayashi N, et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol 1999; 34: 651-659.
- 16. Ge L, Iakovou I, Sangiorgi GM, et al. Treatment of saphenous vein graft lesions with drug-eluting stents: immediate and midterm outcome. J Am Coll Cardiol 2005; 45: 989-994.
- 17. Nakamura S, Muthusamy TS, Bae JH, et al. Impact of sirolimus-eluting stent on the outcome of patients with chronic total occlusions. Am J Cardiol 2005; 95: 161-166.
- 18. Tanabe K, Hoye A, Lemos PA, et al. Restenosis rates following bifurcation stenting with sirolimus-eluting stents for de novo narrowings. Am J Cardiol 2004; 94: 115-118.
- 19. Mittal S, Weiss DL, Hirshfeld JW Jr, et al. Comparison of outcome after stenting for de novo versus restenotic narrowings in native coronary arteries. Am J Cardiol 1997; 80: 711-715.
- 20. Kastrati A, Schomig A, Elezi S, et al. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol 1997; 30: 1428-1436.
- 21. Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol 1998; 32: 584-589.
- 22. Schampaert E, Cohen EA, Schluter M, et al; C-SIRIUS Investigators. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol 2004; 43: 1110-1115.
- 23. Schofer J, Schluter M, Gershlick AH, et al; E-SIRIUS Investigators. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet 2003; 362: 1093-1099.
- 24. Kastrati A, Mehilli J, von Beckerath N, et al; ISAR-DESIRE Study Investigators. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA 2005; 293: 165-171.
- 25. Dibra A, Kastrati A, Mehilli J, et al; ISAR-DIABETES Study Investigators. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med 2005; 353: 663-670.
- 26. Marzocchi A, Piovaccari G, Manari A, et al. Comparison of effectiveness of sirolimus-eluting stents versus bare metal stents for percutaneous coronary intervention in patients at high risk for coronary restenosis or clinical adverse events. Am J Cardiol 2005; 95: 1409-1414.
- 27. Cohen DJ, Bakhai A, Shi C, et al; SIRIUS Investigators. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial. Circulation 2004; 110: 508-514.
- 28. Ellis SG, Bajzer CT, Bhatt DL, et al. Real-world bare metal stenting: identification of patients at low or very low risk of 9-month coronary revascularization. Catheter Cardiovasc Interv 2004; 63: 135-140.
- 29. Lord SJ, Howard K, Allen F, et al. A systematic review and economic analysis of drug-eluting coronary stents available in Australia. Med J Aust 2005; 183: 464-471. <MJA full text>
- 30. Lemos PA, Saia F, Hofma SH, et al. Short- and long-term clinical benefit of sirolimus-eluting stents compared with conventional bare stents for patients with acute myocardial infarction. J Am Coll Cardiol 2004; 43: 704-708.
- 31. Sawhney N, Moses JW, Leon MB, et al. Treatment of left anterior descending coronary artery disease with sirolimus-eluting stents. Circulation 2004; 110: 374-379.
- 32. Dangas G, Ellis SG, Shlofmitz R, et al; TAXUS-IV Investigators. Outcomes of paclitaxel-eluting stent implantation in patients with stenosis of the left anterior descending coronary artery. J Am Coll Cardiol 2005; 45: 1186-1192.
- 33. Lee MS, Kapoor N, Jamal F, et al. Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol 2006; 47: 864-870.
- 34. Tanigawa J, Sutaria N, Goktekin O, Di Mario C. Treatment of unprotected left main coronary artery stenosis in the drug-eluting stent era. J Interv Cardiol 2005; 18: 455-465.





Abstract
Objective: We aimed to assess the pattern of use of drug-eluting stents (DESs) in patients undergoing percutaneous coronary interventions (PCIs) in Victorian public hospitals.
Design, setting and patients: Prospective study comparing the use of one or more DESs versus bare-metal stents (BMSs) only, in consecutive patients undergoing 2428 PCIs with stent implantation from 1 April 2004 to 31 December 2005 at seven Victorian public hospitals.
Main outcome measures: Adherence to current Victorian Department of Human Services guidelines which recommend DES use in patients with high-risk features for restenosis (diabetes, small vessels, long lesions, in-stent restenotic lesions, chronic total occlusions and bifurcation lesions).
Results: Of the 2428 PCIs performed, at least one DES was implanted in 1101 (45.3%) and BMSs only were implanted in 1327 (54.7%). In 87.7% (966/1101) of PCI with DESs, there was at least one criterion for high risk of restenosis. DESs were more likely to be used in patients with diabetes (risk ratio [RR], 2.45; 95% CI, 2.02–2.97), small vessels (RR, 3.35; 95%CI, 2.35–4.76), long lesions (RR, 3.87; 95% CI, 3.23–4.65), in-stent restenotic lesions (RR, 3.98; 95%CI, 2.67–6.06), chronic total occlusions (RR, 1.30; 95% CI, 0.51–2.88) and bifurcation lesions (RR, 2.23; 95%CI, 1.57–3.17). However, 66.2% (1608/2428) of all PCIs were in patients eligible for DESs according to Victorian guidelines, and in 39.9% (642/1608) of these PCIs, a BMS was used.
Conclusion: In Victorian public hospitals, DESs have been largely reserved for patients at high risk of restenosis in accordance with Department of Human Services guidelines. However, many patients with high-risk criteria for restenosis did not receive DESs. Greater use of DESs in these patients may improve outcomes by reducing the need for repeat revascularisation.