We report a case of compassionate use of a haemoglobin-based oxygen carrier in a severely injured Jehovah’s Witness patient, for whom survival was considered unlikely. Severe anaemia and cardiac hypoxia were reversed after slow infusion of this agent. No vasoactive side effects were associated with the treatment, possibly due to the slow infusion, and the patient survived. (MJA 2011; 194: 471-473)
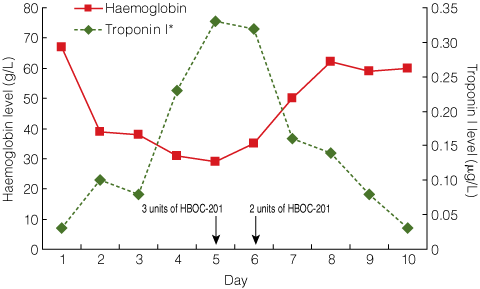
By Day 5, the patient’s Hb level had dropped to 29 g/L and her serum troponin I level was 0.33 μg/L (RR, < 0.10 μg/L), indicating cardiac hypoxia (Box). An electrocardiogram showed widespread ST depression and an episode of non-sustained ventricular tachycardia was documented. Survival with this degree of metabolic demand, the associated anaemia, and resultant end-organ hypoxia was considered unlikely.
Following advice from experienced United States physicians, 3 units of HBOC-201 were administered on Day 5, and a further 2 units were administered on Day 6 with ascorbic acid (1 g twice daily continued until discharge). Each unit of HBOC-201 was infused over 8 hours to minimise any adverse effects related to volume overload, vasoactivity or methaemoglobin. Intravenous glyceryl trinitrate was the agreed treatment in the event of hypertension,1 but this was not necessary.
After the slow administration of 5 units of HBOC-201, the patient’s Hb level increased from 35 g/L to 62 g/L (Box). Echocardiography performed before and after HBOC-201 treatment showed a reduction in cardiac output from 6.8 L/min to 5.0 L/min. Electrocardiography findings and troponin I levels returned to normal and no further arrhythmias were noted. Somatosensory evoked potentials revealed intact lower-limb neurological pathways. On Day 7, closed reduction was performed and a body cast was applied to treat the T12/L1 fracture dislocation. Imaging showed improved alignment, and an inferior vena cava filter was placed.
Haemorrhagic shock is responsible for one-third of deaths following high-energy trauma.2 Integrated trauma care systems coordinate rapid haemorrhage control, shock recognition and surgical interventions to minimise blood loss and coagulopathy.3
Healthy volunteers can tolerate Hb levels of 50 g/L without evidence of end-organ hypoxia.4 However, it is estimated that the median Hb concentration associated with mortality is about 25 g/L.5 During the phase of increased metabolic demand in our patient, there was evidence of cardiac hypoxia when her Hb level reached 29 g/L. This prevented further operative interventions and placed her at high risk of cardiac dysrhythmias and death.
HBOC-201 is a modified lactated Ringer’s solution containing 130 g/L of polymerised Hb of bovine origin. It is compatible with all blood types, stable for 3 years when stored at 2–30°C and stable for 2 years when stored at 40°C. When fully saturated, HBOC-201 has the same oxygen-carrying capacity as whole blood with the same Hb concentration. The partial pressure of oxygen at which HBOC-201 is 50% saturated (40 mmHg) is higher than that for cellular Hb (27 mmHg), which facilitates oxygen delivery to tissues. The half-life of HBOC-201 is approximately 20 hours.6 Polymerisation of the Hb reduces its glomerular diffusion and nephrotoxicity. A potential complication of HBOC-201 administration is hypertension and increased left ventricular afterload. Infusing each unit slowly (over 8 hours) in our patient may have diminished any vasoactive side effects.
Two case reports of using HBOC-201 to treat severe anaemia following blunt trauma have been published. The first described improved cerebral oxygen delivery, but not survival, in a patient with head injuries.7 The second described successful reversal of haemorrhagic shock in a patient whose Hb level dropped to 45 g/L before HBOC-201 administration.8 However, the lack of clear HBOC-201 transfusion indications and end points, as well as the lack of data to support widespread use of HBOCs, has been criticised.9
A meta-analysis of data from HBOC trials has demonstrated an increased incidence of myocardial infarction and death in anaemic patients without life-threatening haemorrhagic shock.10 However, the analysis did not address the issue of “risk versus benefit” for use of these agents, including HBOC-201, in cases where blood transfusion for severely anaemic patients is not possible. A subsequent series of 54 consenting non-trauma patients with a median Hb level of 40 g/L demonstrated improved chances of survival with no serious adverse events following HBOC-201 administration.11
When blood transfusion is not possible, HBOCs can sustain oxygen delivery to hypoxic tissues.12 Such treatment may represent a life-saving intervention for patients with acute anaemia.13
Provenance: Not commissioned; externally peer reviewed.
- 1. Katz LM, Manning JE, McCurdy S, et al. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation 2010; 81: 481-487.
- 2. Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010; 34: 158-163.
- 3. Atkin C, Freedman I, Rosenfeld JV, et al. The evolution of an integrated State Trauma System in Victoria, Australia. Injury 2005; 36: 1277-1287.
- 4. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279: 217-221.
- 5. Weiskopf RB. Emergency transfusion for acute severe anemia: a calculated risk. Anesth Analg 2010; 111: 1088-1092.
- 6. Corporation B. Hemopure. Cambridge, Mass: Biopure Corporation, 2008. http://www.hemopure.co.za/downloads/HemopureSouthAfrica_PI.pdf (accessed Mar 2011).
- 7. Marinaro J, Smith J, Tawil I, et al. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion 2009; 49: 2054-2059.
- 8. Mackenzie CF, Morrison C, Jaberi M, et al. Management of hemorrhagic shock when blood is not an option. J Clin Anesth 2008; 20: 538-541.
- 9. Sarani B, Pryor J. Hemoglobin-based oxygen carriers as rescue therapy: justified experiment or unnecessary risk [editorial]? J Clin Anesth 2008; 20: 489-491.
- 10. Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 2008; 299: 2304-2312.
- 11. Mackenzie CF, Moon-Massat PF, Shander A, et al. When blood is not an option: factors affecting survival after the use of a hemoglobin-based oxygen carrier in 54 patients with life-threatening anemia. Anesth Analg 2010; 110: 685-693.
- 12. Mackenzie CF. Haemoglobin-based oxygen carriers: is the benefit worth the risk? Br J Hosp Med (Lond) 2009; 70: 26-30.
- 13. Donahue LL, Shapira I, Shander A, et al. Management of acute anemia in a Jehovah’s Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion 2010; 50: 1561-1567.





We thank Jeffrey Box (Senior Scientist, Clinical Biochemistry, Alfred Pathology Service) for advice and data related to potential effects of HBOC-201 on serum troponin I analysis.
Mark Fitzgerald had travel and accommodation expenses covered by Biopure (the then manufacturer of HBOC-201) for attendance at a 2-day meeting in Boston in 2006 to provide independent commentary on a planned research project.