Vitamin D deficiency in pregnancy is common, particularly in dark-skinned and veiled women living in temperate climates.1,2 Potential short-term consequences include neonatal hypocalcaemia and seizures. Longer-term complications for offspring include failure to thrive and rickets, which is undergoing a resurgence in Australia.3,4 In adults, deficiency is epidemiologically associated with increased risk of some cancers, autoimmune diseases (including type 1 diabetes) and infectious diseases.5 A number of reports suggest an association between vitamin D deficiency and type 2 diabetes.6-10
Pregnancy is an insulin-resistant state during which improved β-cell function and proliferation normally occur to meet increased secretory demands.11 If, as evidence suggests, vitamin D contributes to insulin sensitivity, or β-cell function and insulin secretion,12 deficiency may contribute to impaired glucose tolerance during pregnancy.
A few studies have explored the relationship between vitamin D status and gestational diabetes mellitus (GDM).13-17 Of these, three examined the relationship between 25-hydroxyvitamin D (25[OH]D) and glucose levels during oral glucose tolerance testing (OGTT), reporting mixed results.13,14,17
GDM was diagnosed according to Australasian Diabetes in Pregnancy Society (ADIPS) criteria:18 a fasting blood glucose level (BGL) of ≥ 5.5 mmol/L and/or a 2-hour BGL of ≥ 8 mmol/L on 75 g OGTT (performed for women with previous GDM and those with positive 50 g glucose challenge test results on routine screening at 26–28 weeks). All women with GDM were seen by a diabetes educator and dietician and were taught home blood glucose monitoring. Commencement of insulin therapy and treatment targets were based on ADIPS recommendations.18
Vitamin D deficiency was defined conservatively as < 25 nmol/L 25(OH)D, insufficiency as 25–50 nmol/L 25(OH)D and sufficiency as > 50 nmol/L 25(OH)D. As many authors recommend higher cut-offs, we also examined the data using 25(OH)D levels of < 50 nmol/L (deficiency), 50–75 nmol/L (insufficiency) and > 75 nmol/L (sufficiency), using recommendations based on the serum parathyroid hormone nadir at 25(OH)D of 75–80 nmol/L.19
The study was approved by the Sydney West Area Health Service Human Research Ethics Committee.
Factors associated with 25(OH)D levels are shown in Box 1. Levels of 25(OH)D differed by ethnicity. Bonferroni-corrected post-hoc analysis revealed comparable levels in the Indian Subcontinental and Middle Eastern groups, which were significantly lower than those for the East or South-East Asian and Caucasian groups (levels in the latter two groups were also comparable). There was also significant seasonal variation in 25(OH)D levels. Levels during summer were significantly higher than those for other seasons, which were comparable on Bonferroni-corrected post-hoc analysis. Women in paid employment had higher mean 25(OH)D levels than those performing home duties. There was no significant association between BMI at booking in and 25(OH)D level.
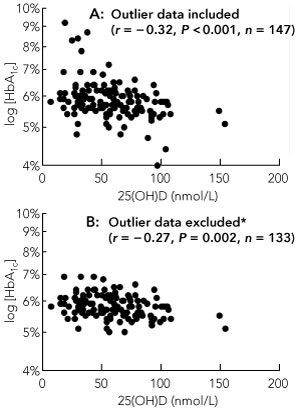
25(OH)D was inversely associated with both fasting and 2-hour BGLs during OGTT (Spearman r = − 0.16; P = 0.05 for both), but not with total daily insulin dose. There was a highly significant inverse association between 25(OH)D levels and log[HbA1c] (Spearman r = − 0.32; P < 0.001) (Box 2). The relationship remained significant after removal of HbA1c outliers that were > 7.5% (P < 0.001) and > 7% (P = 0.008). It also remained significant after removal of HbA1c values that were ≥ 6% (P = 0.02), even though this excluded 38% (56) of the study sample. Excluding both low (< 5%) and high (> 7%) HbA1c outliers also did not negate the relationship (r = − 0.27; P = 0.002) (Box 2).
Vitamin D insufficiency has a well established impact on bone density, neonatal vitamin D and calcium status, and childhood rickets.3-5,20,21 The 41% prevalence of inadequate 25(OH)D levels in women with GDM in our study is unacceptable and identifies vitamin D insufficiency as an issue of public health significance. The high rates of deficient and insufficient 25(OH)D levels occurred despite all women being advised to take pregnancy multivitamins containing either 400 IU or 500 IU cholecalciferol per day and despite the temperate climate of Sydney.
Previous studies provide mechanistic support for these findings. Mouse models of vitamin D deficiency demonstrate altered insulin secretion.19 In a study of pregnant Iranian women, 25(OH)D levels were inversely correlated with homeostasis model of assessment for insulin resistance (HOMA-IR) index, suggesting that deficiency may contribute to insulin resistance.15 In women with GDM, acute administration of intravenous 1,25-hydroxyvitamin D has been shown to decrease fasting glucose levels without altering the glucose response to OGTT, although insulin levels were decreased; it was concluded that 1,25-hydroxyvitamin D might have effects through altered insulin sensitivity.16
Our findings support a previously reported inverse correlation between 25(OH)D and fasting glucose, insulin and HOMA-IR index, measured during OGTT screening in obstetric clinic attendees; after adjusting for ethnicity, age and BMI, the relationship with glucose retained borderline significance.13 In our study, while age, ethnicity, season and occupation significantly affected 25(OH)D levels, they showed no significant relationship with log[HbA1c] either alone or in multivariable linear models. The lack of association between 25(OH)D and BMI concurs with the results of other studies of vitamin D in pregnancy, suggesting that body weight is unlikely to be a major confounder. As our study was retrospective, other potential confounders (eg, sunlight exposure, physical activity, dietary vitamin D and calcium intake) could not be examined.
A recent study of Indian women failed to demonstrate a relationship between vitamin D status and risk of developing GDM, although in mothers with 25(OH)D levels < 50 nmol/L, higher 25(OH)D concentrations were associated with lower 30-minute glucose levels on OGTT and higher fasting proinsulin concentrations.14 Population differences may have contributed to variation in these results. The mean vitamin D data in this study had much larger variation by season than in our study (< 30 nmol/L to > 70 nmol/L depending on the month), suggesting that levels over the course of pregnancy may have been relatively unstable.
Genetic variants of 25-hydroxylase, 1-α-hydroxylase and the vitamin D receptor have been described in patients with GDM.22 A combination of genetic and environmental factors determine vitamin D levels and their effects at the β-cell or insulin-sensitive tissues.
The relationship between 25(OH)D and severity of glycaemia demonstrated in our study is in the context of existing GDM. The results suggest that the possible contribution of vitamin D status during pregnancy to the pathogenesis of glucose intolerance in pregnancy should be studied further. In the Hyperglycemia and Adverse Pregnancy Outcomes study,23 an increase of 0.4 mmol/L of fasting glucose corresponded to an odds ratio of 1.38 for birthweight > 90th centile for the offspring, an odds ratio of 1.55 for neonatal cord blood C-peptide > 90th centile and an odds ratio of 1.11 for caesarean delivery (all of which were significant). An increase in 1-hour glucose of 1.7 mmol/L corresponded to odds ratios for the same outcomes of 1.46, 1.46 and 1.10, respectively. Compared to women with adequate 25(OH)D levels (> 50 nmol/L), women with inadequate levels demonstrated differences of 0.4 mmol/L in fasting glucose, 2.4 mmol/L in 1-hour glucose and 0.4% in HbA1c.These findings suggest that the size of the potential effect of vitamin D level is clinically highly relevant.
About 6%–10% of pregnant women in Australia develop GDM,24 which has long-term implications for their future risk of type 2 diabetes and also for the health of their offspring. Maternal diabetes in pregnancy increases the risk of obesity and diabetes in the offspring. Thus, if increasing 25(OH)D levels reduced the incidence or severity of GDM, this could have profound public health significance. The possibility that vitamin D supplementation might reduce gestational glycaemia is an intriguing hypothesis which warrants testing in prospective randomised clinical trials.
In our study population, there was an unacceptably high prevalence of vitamin D insufficiency and deficiency. Considering this in conjunction with the strong correlation of maternal 25(OH)D with cord blood and neonatal 25(OH)D1 and the known risks for neonates associated with low maternal 25(OH)D, we suggest routine testing of all pregnant women at the time of screening for GDM or earlier, and treatment of women who are found to be vitamin D deficient. Our study also revealed a potential link between vitamin D status and GDM, which should be studied further.
Provenance: Not commissioned; externally peer reviewed.
Received 8 September 2009, accepted 15 December 2010
- Sue Lynn Lau1,2,3
- Jenny E Gunton1,2,3,4
- Neil P Athayde1,3
- Karen Byth1
- N Wah Cheung1,3
- 1 Westmead Hospital, Sydney, NSW.
- 2 Diabetes and Transcription Factors Laboratory, Garvan Institute of Medical Research, Sydney, NSW.
- 3 Western Clinical School, University of Sydney, Sydney, NSW.
- 4 St Vincent’s Clinical School, University of New South Wales, Sydney, NSW.
Sue Lynn Lau was supported by the Royal Australasian College of Physicians (RACP), the National Health and Medical Research Council (NHMRC) and a Diabetes Australia Research Trust grant. Jenny Gunton was supported by the NHMRC, the Juvenile Diabetes Research Foundation, the RACP and the Diabetes Australia Research Trust.
None identified.
- 1. Bodnar LM, Simhan HN, Powers RW, et al. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007; 137: 447-452.
- 2. Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child 2007; 92: 737-740.
- 3. Munns C, Zacharin MR, Rodda CP, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 2006; 185: 268-272. <MJA full text>
- 4. Robinson PD, Högler W, Craig ME, et al. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child 2006; 91: 564-568.
- 5. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008; 87: 1080S-1086S.
- 6. Norman AW, Frankel BJ, Helt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980; 209: 823-825.
- 7. Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986; 29: 142-145.
- 8. Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non insulin dependent diabetes mellitus. Am J Clin Nutr 1994; 59: 1083-1087.
- 9. Boucher BJ, Mannan N, Noonan K, et al. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 1995; 38: 1239-1245.
- 10. Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984; 73: 759-766.
- 11. Van Assche FA, Holemans K, Aerts L. Long-term consequences for offspring of diabetes during pregnancy. Br Med Bull 2001; 60: 173-182.
- 12. Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008; 10: 185.
- 13. Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet Med 2008; 25: 678-684.
- 14. Farrant HJW, Krishnaveni GV, Hill JC, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr 2008; 63: 646-652.
- 15. Maghbooli Z, Hossein-Nezhad A, Karimi F, et al. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev 2008; 24: 27-32.
- 16. Rudnicki PM, Molsted-Pedersen L. Effect of 1,25-dihydroxycholecalciferol on glucose metabolism in gestational diabetes mellitus. Diabetologia 1997; 40: 40-44.
- 17. Zhang C, Qui C, Hu FB, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One 2008; 3: e3753.
- 18. Hoffman L, Nolan C, Wilson JD, et al. Gestational diabetes mellitus — management guidelines. Med J Aust 1998; 169: 93-97. <MJA full text>
- 19. Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005; 90: 3215-3224.
- 20. Wharton B, Bishop N. Rickets. Lancet 2003; 362: 1389-1400.
- 21. Nozza JM, Rodda CP. Vitamin D deficiency in mothers of infants with rickets. Med J Aust 2001; 175: 253-255. <MJA full text>
- 22. Ramos-Lopez E, Jansen T, Ivaskevicius V, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci 2006; 1079: 327-334.
- 23. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991-2002.
- 24. Cheung NW, Byth K. The population health significance of gestational diabetes. Diabetes Care 2003; 26: 2005-2009.





Abstract
Objective: To test the hypothesis that lower 25-hydroxyvitamin D (25[OH]D) levels in late pregnancy are associated with poorer glucose control in gestational diabetes mellitus (GDM).
Design and setting: Retrospective cross-sectional study, in a GDM clinic at a tertiary referral centre.
Patients: Women attending the GDM clinic at Westmead Hospital from 1 February 2007 to 1 February 2008, excluding those with prepregnancy glucose intolerance.
Main outcome measures: Levels of glycated haemoglobin (HbA1c) and 25(OH)D measured during the third trimester; maternal age, ethnicity, body mass index (BMI) and occupational status; and results of oral glucose tolerance testing (OGTT).
Results: 147 women with a mean gestational age of 35 ± 2 weeks were included, of whom 41% had insufficient or deficient levels of 25(OH)D (≤ 50 nmol/L). Ethnicity, occupational status and season significantly influenced 25(OH)D levels (P < 0.01 for all) but BMI did not. 25(OH)D levels were inversely associated with fasting and 2-hour blood glucose levels during OGTT (Spearman r = − 0.16; P = 0.05 for both) and with log[HbA1c] (Spearman r = − 0.32; P < 0.001). BMI and insulin doses were also associated with HbA1c levels. Multivariable analysis identified 25(OH)D and blood glucose levels during the OGTT as independent predictors of HbA1c levels.
Conclusions: Lower 25(OH)D levels are independently associated with poorer glycaemic control. Future randomised trials are needed to determine whether vitamin D plays a role in glycaemic control in GDM. Regardless, maternal vitamin D insufficiency has adverse effects including neonatal hypocalcaemia and rickets. The 41% prevalence of inadequate 25(OH)D levels in the women in our study is unacceptably high. We propose routine 25(OH)D testing of all pregnant women at screening for GDM or earlier, and treatment of women who are found to be deficient.