The prevalence of diabetes is 7.4% in Australia.1 This continues to increase with an annual incidence of 0.8% in adult Australians.1 Diabetes is associated with excess mortality, major morbidities and substantial use of health care resources.2 The early phase of diabetes is usually asymptomatic and can remain undetected for many years, with about half of these patients unaware they have the condition.1,3 Treatment of diabetes, hypertension and dyslipidaemia reduces long-term complications.3-5 These factors favour early detection of diabetes by screening.6,7
The prevalence of diabetes among hospitalised patients is about three times the community prevalence.1,8,9 Furthermore, hospitalisation is a point of contact with the health care system. However, this opportunity for population screening is not currently recommended because glucose-based tests are confounded by stress hyperglycaemia.10
Screening for diabetes by measuring fasting plasma glucose (FPG) and/or using oral glucose tolerance tests (OGTTs) among high-risk individuals has been recommended since 1965.11 Glycated haemoglobin (HbA1c) testing was recently recommended by the International Expert Committee as the preferred test to diagnose diabetes and this has been adopted by the American Diabetes Association (ADA).6,12 Diabetes defined by HbA1c level predicts microvascular and macrovascular complications at least as well as diabetes defined by glucose testing.3,4,13-15 HbA1c testing is familiar to clinicians, as it is routinely used in clinical practice to guide treatment, and is the preferred biochemical marker of diabetes in clinical studies. HbA1c levels are less affected by acute illness than plasma glucose levels, and are therefore more likely to be reliable in the hospital setting.16 We hypothesised that HbA1c levels could be used as an automated screening test in hospitalised patients for the diagnosis of diabetes.
This project was approved by the Flinders Clinical Research Ethics Committee. A prospective, observational study of all adult non-obstetric patients admitted to a tertiary teaching hospital in South Australia from 1 April to 30 June 2009 was conducted. For all patients, a random plasma glucose (RPG) level ≥ 5.5 mmol/L on admission automatically triggered an HbA1c test on the first blood sample drawn during routine clinical care (Box 1). Patients admitted more than once during the study period were assessed only on the basis of the first admission with RPG level ≥ 5.5 mmol/L.
Patients were categorised as having known diabetes, undiagnosed diabetes or no diabetes. A patient was classified as having known diabetes if their discharge diagnoses included International Classification of Diseases (ICD-10) codes E10–E14. Patients were defined as having undiagnosed diabetes if they were not coded as having diabetes and their HbA1c level was ≥ 6.5% (48 mmol/mol), based on the International Expert Committee recommendation.12 The HbA1c results of patients with undiagnosed diabetes were provided to their general practitioner.
Cost analysis was based on the cost of performing an HbA1c laboratory test, which was $16.90 at the time of the study.17 RPG tests were performed on all patients included in the study as part of routine clinical care, and were not included in cost analysis. The cost analysis was extended to include patients with RPG < 5.5 mmol/L (excluded from the study) using the conservative assumption that none of these patients had undiagnosed diabetes. The cost of screening all hospitalised patients for diabetes was estimated based on the community incidence of diabetes (0.8% per year) and assuming half are undiagnosed.1
HbA1c testing was conducted among 2672 patients. The study group was 93% white, comprised 48% women, and ranged in age from 18 to 102 years. The characteristics of the three groups classified by diabetes status are shown in Box 2. The prevalence of diabetes was 21.5% (95% CI, 19.9%–23.0%) among patients admitted to hospital with RPG ≥ 5.5 mmol/L. Diabetes was previously undiagnosed among 262/2360 patients (11.1%; 95% CI, 9.8%–12.4%). There were 312 patients with known diabetes (11.7%; 95% CI, 10.5%–12.9%). The prevalence of undiagnosed diabetes was highest in the 65–74-years age group (Box 3). In our study, the prevalence of diabetes among hospitalised patients was three times higher than the community prevalence. Among the younger patients (25–34 years), the hospital prevalence of diabetes was 24 times higher than the community prevalence (Box 3).
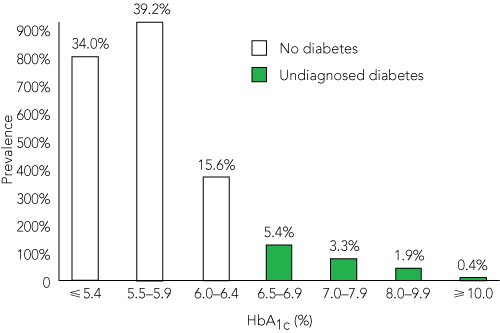
The HbA1c levels of patients with no or undiagnosed diabetes are shown in Box 4. In addition to the 11% with unknown diabetes, a further 35% (822) had HbA1c levels between 5.7% and 6.4%, classified as “increased risk for diabetes” by the ADA.6 Among patients with no or undiagnosed diabetes, RPG levels ≥ 11.1 mmol/L (World Health Organization definition) had a sensitivity of 28% and a specificity of 98% to diagnose diabetes. The area under the ROC curve for RPG level as a diagnostic test for diabetes was 0.78 (95% CI, 0.75–0.81).
According to Australian guidelines,18 66/262 patients (25%) with undiagnosed diabetes and 422/2098 patients (20%) with no diabetes met criteria for screening, with a positive predictive value of 14% and a negative predictive value of 90% for patients with undiagnosed diabetes. Seventeen per cent of patients with undiagnosed diabetes indicated they do not have a regular GP.
We found that a high proportion of hospitalised patients with a RPG level ≥ 5.5 mmol/L had undiagnosed diabetes. The prevalences of known and undiagnosed diabetes were about threefold higher than in the Australian community.1,8,9 Among hospitalised patients younger than 55 years, the prevalence of diabetes was almost fivefold higher than in the community. We used the recent International Expert Committee recommended diagnostic threshold of HBA1c levels ≥ 6.5% to detect undiagnosed diabetes among hospitalised patients. A previous cohort study of unselected emergency admissions, which defined diabetes as an HbA1c level ≥ 6.2%, reported that 18% of patients had an HbA1c level above this value.8 In our study, 17% of patients had an HbA1c level ≥ 6.2%.
HbA1c monitoring can be used as an automated screening test in hospitalised patients using blood samples drawn during usual clinical care. Potential benefits of an automated HbA1c screening program include low implementation costs and extensive coverage of a high-risk group. In contrast, glucose-based automated screening processes are unreliable due to unknown fasting status and stress hyperglycaemia.8 Automated HbA1c testing was easily implemented using existing blood samples and was an inexpensive method to diagnose diabetes in this patient cohort.
Although RPG testing is not recommended for diagnosing diabetes, evidence suggests RPG testing is commonly used for reasons of convenience.5 In our study, RPG levels had low sensitivity in diagnosing diabetes; using the recommended RPG level of ≥ 11.1 mmol/L would have missed 188 patients with diabetes. Using lower thresholds improves sensitivity, but compromises specificity. This is consistent with many other studies.8,16,19
Other studies used FPG testing and/or OGTT to diagnose diabetes in hospitalised patients and reported rates of undiagnosed diabetes from 2.6% to 9%.20,21 The lower rates of diabetes in some studies may reflect the poor sensitivity of fasting glucose in this setting. We did not assess FPG levels in this study, but demonstrated that a post-discharge OGTT is unlikely to be an effective screening method. Despite a personal approach and two reminders, the completion rate of OGTTs was only 27%. Although other published studies have reported a higher completion rate (55%–58%), they have also noted poor compliance with this test.22,23
There is agreement about the need for screening and early intervention of diabetes. There is currently no recommendation to screen hospitalised patients in international guidelines.6,7,18 Existing Australian screening guidelines would have led to testing of only 25% of patients with unknown diabetes in our study. This is consistent with previous reports that established risk factors are poor predictors of diabetes or impaired glucose tolerance among hospitalised patients.19 Furthermore, 17% of patients with unknown diabetes in this study did not identify having a regular GP. This highlights the value of using hospitalisation as a point of contact with the health care system to screen patients who would otherwise be missed.
There are limitations to the use of HbA1c testing among hospitalised patients, particularly disorders of increased red cell turnover (eg, haemolytic disorders) and blood transfusion. However, these conditions are unlikely to be present in numbers that affect overall screening, and “first samples” are likely to be before transfusion.3
1 Enrolment of study participants
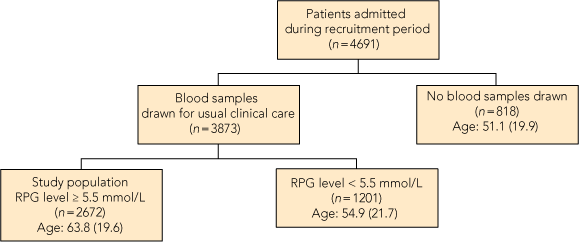 Age is presented in mean (SD) years. RPG = random plasma glucose. |
2 Characteristics of hospital patients whose glycated haemoglobin levels were tested, according to diagnostic status*
3 Prevalence of diabetes among hospitalised patients, by age
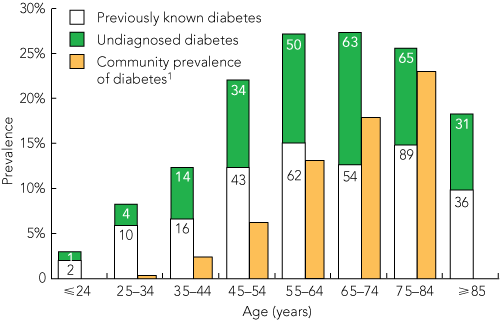 The number in each group is shown within the columns. |
Received 14 May 2010, accepted 3 November 2010
- Nyoli A Valentine1,2
- Tariq M Alhawassi3
- Greg W Roberts4
- Parind P Vora5
- Stephen N Stranks5
- Matthew P Doogue2,5
- 1 Sturt Fleurieu General Practice Education and Training, Adelaide, SA.
- 2 Discipline of Clinical Pharmacology, Flinders University, Adelaide, SA.
- 3 College of Pharmacy, Department of Clinical Pharmacy, King Saud University, Riyadh, Saudi Arabia.
- 4 Repatriation General Hospital, Adelaide, SA.
- 5 Southern Adelaide Diabetes and Endocrine Service, Southern Adelaide Health Service, Adelaide, SA.
Nyoli Valentine was supported by Sturt Fleurieu General Practice Education and Training, South Australia. We acknowledge the assistance of Fotios Visvardis and Emanuel Saris for laboratory support, David Fechner for information technology support, and Richard Woodman and Camille Schubert for statistical and economic advice, respectively.
Nyoli Valentine was supported by Sturt Fleurieu General Practice Education and Training, South Australia. We acknowledge the assistance of Fotios Visvardis and Emanuel Saris for laboratory support, David Fechner for information technology support, and Richard Woodman and Camille Schubert for statistical and economic advice, respectively.
- 1. Barr E, Magliano D, Zimmet P, et al. AusDiab 2005. The Australian Diabetes, Obesity and Lifestyle Study. Tracking the accelerating epidemic: its causes and outcomes. Report. Melbourne: International Diabetes Institute, 2006.
- 2. Roper NA, Bilous RW, Kelly WF, et al. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001; 322: 1389-1393.
- 3. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med 2007; 24: 333-343.
- 4. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837-853.
- 5. Ealovega MW, Tabaei BP, Brandle M, et al. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care 2004; 27: 9-12.
- 6. American Diabetes Association. Standards of medical care in diabetes — 2009. Diabetes Care 2009; 32 Suppl 1: S13-S61.
- 7. Screening for type 2 diabetes. Report of a World Health Organization and International Diabetes Federation meeting. Geneva: World Health Organization, 2003.
- 8. Wexler DJ, Nathan DM, Grant RW, et al. Prevalence of elevated hemoglobin A1c among patients admitted to the hospital without a diagnosis of diabetes. J Clin Endocrinol Metab 2008; 93: 4238-4244.
- 9. Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabet Med 2008; 25: 1157-1163.
- 10. Greci LS, Kailasam M, Malkani S, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care 2003; 26: 1064-1068.
- 11. World Health Organization. Diabetes mellitus, a report of the WHO Expert Committee. WHO Technical Report Series No. 310. Geneva: WHO, 1965.
- 12. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327-1334.
- 13. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977-986.
- 14. Sabanayagam C, Liew G, Tai ES, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia 2009; 52: 1279-1289.
- 15. Tapp RJ, Tikellis G, Wong TY, et al. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008; 31: 1349-1354.
- 16. Saudek CD, Herman WH, Sacks DB, et al. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008; 93: 2447-2453.
- 17. Australian Government, Department of Health and Ageing. Medical Benefits Schedule. DoHA, 2009.
- 18. Colagiuri S, Zimmet P, Hepburn A, Colagiuri R. Evidence based guidelines for type 2 diabetes: primary prevention, case detection and diagnosis. Canberra: Diabetes Australia and National Health and Medical Research Council, 2002.
- 19. Krebs JD, Robinson GM, Smith RB, et al. Follow up testing of hyperglycaemia during hospital admission: combined use of fasting plasma glucose and HbA1c. N Z Med J 2000; 113: 379-381.
- 20. Baker ST, Chiang CY, Zajac JD, et al. Outcomes for general medical inpatients with diabetes mellitus and new hyperglycaemia. Med J Aust 2008; 188: 340-343.
- 21. George PM, Valabhji J, Dawood M, et al. Screening for Type 2 diabetes in the accident and emergency department. Diabet Med 2005; 22: 1766-1769.
- 22. Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002; 25: 829-834.
- 23. Garancini MP, Calori G, Ruotolo G, et al. Prevalence of NIDDM and impaired glucose tolerance in Italy: an OGTT-based population study. Diabetologia 1995; 38: 306-313.





Abstract
Objective: To assess the utility of glycated haemoglobin (HbA1c) level as an automated screening test for undiagnosed diabetes among hospitalised patients and to estimate the prevalence of undiagnosed diabetes among hospitalised patients.
Design, participants and setting: A 3-month prospective study of all adult patients admitted to a tertiary hospital. An HbA1c test was automatically undertaken on admission for all patients with a random plasma glucose (RPG) level ≥ 5.5 mmol/L. Demographic, admission and biochemical data were obtained from hospital databases. A subset of patients was recruited for an oral glucose tolerance test (OGTT) after discharge.
Main outcome measures: Prevalence of undiagnosed diabetes (defined as HbA1c ≥ 6.5% in accordance with International Expert Committee and American Diabetes Association recommendations) and utility of automated HbA1c testing.
Results: The prevalence of undiagnosed diabetes was 11% (95% CI, 9.8%–12.4%) (262/2360) during the study period. A further 312 patients with known diabetes were admitted. The prevalence of undiagnosed diabetes was highest in the 65–74-years age group. The HbA1c test cost was $152 per new diagnosis of diabetes. Conservatively assuming an annual incidence of undiagnosed diabetes of 0.8%, the ongoing cost of testing hospitalised patients would be $2100 per new diagnosis of diabetes. RPG testing was not sensitive or specific in diagnosing diabetes. Patients were poorly compliant with the post-discharge OGTT (27% completion rate).
Conclusions: HbA1c is a simple, inexpensive screening test that can be automated using existing clinical blood samples. Hospital screening for diabetes needs to be coupled with resources for management in the community.