Vitamin D is a steroid prohormone formed in the skin following ultraviolet B radiation. Vitamin D is hydroxylated to form 25-hydroxyvitamin D (25-OHD), the biomarker of vitamin D status. Little vitamin D is obtained through diet. Melanin filters incident ultraviolet B light, such that darker-skinned individuals synthesise less vitamin D than paler-skinned individuals,1 and have lower 25-OHD levels.2,3 Vitamin D is crucial in calcium homeostasis, with direct effects on intestine, bone, muscle4 and numerous other tissues. The vitamin D receptor is virtually ubiquitous in the body, and insufficient vitamin D levels and vitamin D receptor genotype have been implicated in a wide range of health problems.5-9
Little is known about the vitamin D status of Aboriginal Australians. One study found that muscle pain, a potential consequence of vitamin D deficiency, is highly prevalent in Aboriginal people, and that it is often associated with low 25-OHD levels.10 The literature regarding the vitamin D status of other dark-skinned groups, including African Americans,2,11 Pacific Islanders and Maori,3,12 and indigenous Canadians,13 consistently shows that these groups have lower 25-OHD levels than comparable non-indigenous populations.
We investigated the range of 25-OHD levels in a South Australian Aboriginal population and examined the relationship between 25-OHD levels and biochemical variables of calcium and bone mineral homeostasis, as well as factors that may influence vitamin D synthesis, storage and metabolism.
Of 58 participants (40 men, 18 women; mean age, 38.9 years) included in the study, 51 were staff or patients at Nunkuwarrin Yunti, and seven were patients from the Tullawon Health Service.
Demographics and overall mean biochemical variables for our study population are shown in Box 1. Mean levels for biochemical variables, grouped according to serum 25-OHD levels, are set out in Box 2. Serum 25-OHD levels were normally distributed for the bulk of participants, with a maximum level of 111 nmol/L.
Serum 25-OHD levels showed a definite seasonal variation: highest in late summer–autumn, lowest in late winter–spring (Box 3), and significantly higher in summer than in all other months (P < 0.001, analysis of variance [ANOVA]) (Box 4 and Box 5).
Spearman rank correlation coefficients between the measured variables are shown in Box 6. β-CTx levels decreased with age and were inversely related to 25-OHD levels (P = 0.03). Body mass index (BMI) was positively related to fasting glucose (P = 0.002) and PTH levels (P = 0.001), but not related to 25-OHD levels (P = 0.87). Age was also positively related to fasting glucose levels (P = 0.025). There was a non-significant trend towards an association between 25-OHD levels and time spent outdoors (P = 0.058, ANOVA) (data not shown). No significant association was found between 25-OHD level and smoking status (P = 0.91), alcohol consumption level (P = 0.47) or skin colour (P = 0.20) (data not shown).
Our findings suggest that vitamin D insufficiency (serum 25-OHD ≤ 60 nmol/L) is highly prevalent in this population of adult Aboriginal Australians. Mean 25-OHD levels peaked in summer (84.2 nmol/L), but were below 60 nmol/L in other seasons, indicating prolonged periods of vitamin D insufficiency. The overall mean serum 25-OHD level (56.8 nmol/L) was significantly lower than the mean of 76.9 nmol/L reported for a control group of 36 Adelaide residents (mean age, 32 years).14 Others have reported data from non-Indigenous Australians indicating that in winter and spring the proportion of the population with 25-OHD levels less than 50 nmol/L varied between 37.4% (Geelong, latitude 37 °S) and 67.3% (Hobart, 43 °S).15 In our Aboriginal Australian population, 20 of 37 participants (54%) assessed during winter and spring in Adelaide (35 °S) had a level below 50 nmol/L. These data suggest that Aboriginal Australians are likely to have a poorer vitamin D status than non-Indigenous Australians.
The seasonal variation in 25-OHD levels and the trend towards an association between serum 25-OHD levels and time spent outdoors suggest that production of vitamin D in the skin is the principal determinant of 25-OHD levels. These findings are consistent with research findings regarding the overall Australian population16 and African Americans.17 It is likely that time spent outdoors, particularly if it includes weight-bearing exercise, will have health benefits in addition to those associated with increased vitamin D production.
Darker skin pigmentation in Aboriginal Australians probably contributes to their lower mean 25-OHD level compared with that of non-Indigenous Australians. The lack of a significant association between skin colour and serum 25-OHD in our study may reflect the small sample size. We found no significant association between serum 25-OHD levels and BMI, in contrast with other studies that have shown a significant, negative correlation between BMI and serum 25-OHD levels in populations of all pigmentation types.18-20 It is not clear why our study population should differ from others.
The significant association between BMI and PTH levels found in our study has been reported elsewhere, although it is unknown whether elevated serum PTH levels cause obesity, or whether obesity causes elevated serum PTH levels.21
A significant association was found between serum 25-OHD and fasting serum β-CTx levels, but not between serum 25-OHD and PTH levels. Clinical and animal studies have shown that inadequate serum levels of 25-OHD cause a rise in serum PTH levels, providing information on critical 25-OHD levels required to suppress calciotropic hormones and bone turnover markers. In our study, the serum 25-OHD level at which the β-CTx level began to rise (the inflection point) was about 80 nmol/L, which is comparable to that reported previously.22 A slightly lower inflection point for 25-OHD and PTH (40 nmol/L) was reported in African American women.23 We found that β-CTx levels decreased with age. This may be a reflection of higher bone turnover in participants aged about 28 years or younger.
Important limitations of our study were the small sample size, non-random selection process, and self-reporting of events in the month leading up to the study, which is subject to recall bias. While not truly representative of the Aboriginal population as a whole, our study participants included both staff and patients, and excluded people known to have diabetes, which was felt to be a reasonable compromise given the difficulties associated with recruiting a more formally representative sample. Assuming an effect size similar to previously published work, a sample size of 202 participants would have been required to properly examine the relationship between levels of vitamin D and fasting glucose.24,25 Our sample was therefore too small for this purpose, and was also too small to confirm any negative effect of smoking on vitamin D status.26,27
In summary, vitamin D insufficiency is common in this population of adult Aboriginal Australians, as indicated by the low mean serum 25-OHD level overall and low mean levels found in all seasons other than summer. Target serum 25-OHD levels above 60 nmol/L have been recommended for prevention of fractures and falls,28 with the suggestion that higher target levels (75–80 nmol/L) may be required for preventing the proposed immunologic and metabolic consequences of vitamin D deficiency.29 Our study adds to previous work concerning the vitamin D status of dark-skinned, populations and will aid the design of future studies with adequate statistical power.
1 Age, BMI, and levels of 25-OHD, fasting glucose, PTH and fasting β-CTx in 58 Aboriginal adults
Variable |
Minimum |
Maximum |
Mean |
SD |
Reference range |
||||||||||
Age (years) |
18 |
67 |
38.9 |
10.3 |
na |
||||||||||
BMI (kg/m2) |
17.1 |
55.3 |
29.8 |
7.9 |
19–25 (desirable) |
||||||||||
25-OHD (nmol/L) |
20 |
111 |
56.8 |
22.1 |
> 60 (desirable) |
||||||||||
Fasting glucose (mmol/L) |
3.7 |
11.5 |
5.0 |
1.2 |
3.8–5.5 |
||||||||||
PTH (pmol/L) |
0.3 |
13 |
4.2 |
2.3 |
1.1–5.5 |
||||||||||
Fasting β-CTx (ng/L) |
118 |
668 |
337 |
147.7 |
< 400 (desirable) |
||||||||||
BMI = body mass index. β-CTx = C-terminal telopeptides of type 1 collagen. na = not applicable. 25-OHD = 25-hydroxyvitamin D. PTH = parathyroid hormone. |
|||||||||||||||
2 Age, BMI, and levels of fasting glucose, PTH and fasting β-CTx in 58 Aboriginal adults, by serum 25-OHD level
25-OHD level (nmol/L) |
No. |
Variable |
Minimum |
Maximum |
Mean |
Median* |
SD |
IQR* |
|||||||
< 60 |
36 |
Age (years) |
18 |
57 |
36.9 |
— |
9.1 |
— |
|||||||
|
|
BMI (kg/m2) |
19.9 |
55.3 |
31.2 |
29.8 |
8.7 |
11.0 |
|||||||
|
|
Fasting glucose (mmol/L) |
3.9 |
11.5 |
5.1 |
4.7 |
1.5 |
0.8 |
|||||||
|
|
PTH (pmol/L) |
1.7 |
13 |
4.6 |
4.3 |
2.4 |
3.1 |
|||||||
|
|
Fasting β-CTx (ng/L) |
144 |
662 |
363.8 |
362 |
133.7 |
172 |
|||||||
61–80 |
16 |
Age (years) |
24 |
67 |
41.4 |
— |
12.2 |
— |
|||||||
|
|
BMI (kg/m2) |
17.1 |
40.7 |
27.7 |
26.9 |
6.2 |
7.7 |
|||||||
|
|
Fasting glucose (mmol/L) |
3.7 |
5.6 |
4.8 |
5.0 |
0.6 |
0.9 |
|||||||
|
|
PTH (pmol/L) |
1.1 |
8.0 |
3.9 |
3.7 |
2.3 |
2.9 |
|||||||
|
|
Fasting β-CTx (ng/L) |
118 |
668 |
278.5 |
238 |
152.5 |
195 |
|||||||
> 80 |
6 |
Age (years) |
20 |
46 |
35.5 |
— |
12.4 |
— |
|||||||
|
|
BMI (kg/m2) |
21.6 |
37.3 |
28.1 |
28.0 |
5.6 |
6.6 |
|||||||
|
|
Fasting glucose (mmol/L) |
4.1 |
6.2 |
5.0 |
5.0 |
0.7 |
0.4 |
|||||||
|
|
PTH (pmol/L) |
0.3 |
4.2 |
3.0 |
3.6 |
1.5 |
1.7 |
|||||||
|
|
Fasting β-CTx (ng/L) |
135 |
650 |
336.3 |
292.5 |
191.4 |
280 |
|||||||
BMI = body mass index. β-CTx = C-terminal telopeptides of type 1 collagen. IQR = interquartile range. 25-OHD = 25-hydroxyvitamin D. PTH = parathyroid hormone. * Median and IQR only given for variables that were not normally distributed. |
|||||||||||||||
3 Seasonal variation of 25-dehydroxyvitamin D (25-OHD) levels in 58 Aboriginal Australian adults, by sex*
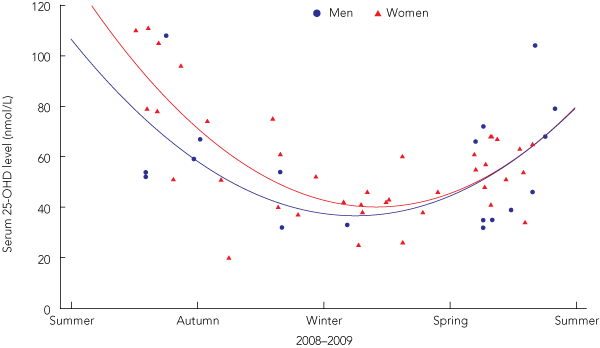 * Data collected from May 2008 to December 2009 and aggregated by season. |
4 Seasonal variation of serum 25-hydroxyvitamin D (25-OHD) levels in 58 Aboriginal adults
Mean 25-OHD level (nmol/L) |
Number |
||||||||||||||
Season |
n |
≤ 25 nmol/L |
26–60 nmol/L |
61–80 nmol/L |
> 80 nmol/L |
||||||||||
Autumn |
10 |
51.1 |
1 |
5 |
4 |
0 |
|||||||||
Winter |
12 |
40.8 |
1 |
11 |
0 |
0 |
|||||||||
Spring |
23 |
52.1 |
0 |
15 |
8 |
0 |
|||||||||
Summer |
13 |
84.2 |
0 |
3 |
4 |
6 |
|||||||||
5 Significance of seasonal differences in 25-hydroxyvitamin D status in 58 Aboriginal adults
|
|
Differences of least squares means |
|||||||||||||
Index season |
Comparator season |
Estimate |
SEM |
df |
t |
P |
|||||||||
Autumn |
Spring |
− 0.05 |
5.98 |
54 |
− 0.01 |
0.994 |
|||||||||
Autumn |
Summer |
− 32.41 |
6.64 |
54 |
− 4.88 |
< 0.001* |
|||||||||
Autumn |
Winter |
11.07 |
6.76 |
54 |
1.64 |
0.108 |
|||||||||
Spring |
Summer |
− 32.37 |
5.67 |
54 |
− 5.71 |
< 0.001* |
|||||||||
Spring |
Winter |
11.11 |
5.81 |
54 |
1.91 |
0.061 |
|||||||||
Summer |
Winter |
43.48 |
6.49 |
54 |
6.70 |
< 0.001* |
|||||||||
df = degrees of freedom. * Significance of correlation: P < 0.05. |
|||||||||||||||
6 Correlation matrix for 25-OHD and age, BMI, and fasting glucose, PTH and fasting β-CTx levels in 58 Aboriginal adults (Spearman rank correlation coefficient)
Variable |
Age |
BMI |
Fasting glucose* |
PTH |
Fasting β-CTx |
||||||||||
BMI |
0.069 |
|
|
|
|
||||||||||
Fasting glucose* |
0.306† |
0.421† |
|
|
|
||||||||||
PTH |
0.129 |
0.428† |
0.143 |
|
|
||||||||||
Fasting β-CTx |
− 0.408† |
− 0.146 |
− 0.041 |
0.183 |
|
||||||||||
25-OHD |
0.08 |
0.02 |
0.196 |
− 0.141 |
− 0.293† |
||||||||||
BMI = body mass index. β-CTx = C-terminal telopeptides of type 1 collagen. 25-OHD = 25-hydroxyvitamin D. PTH = parathyroid hormone. * In all analyses of fasting glucose, values were excluded for two participants whose fasting glucose levels were indicative of the presence of diabetes. † Significance of correlation: P < 0.05. |
|||||||||||||||
- Simon J Vanlint1
- Howard A Morris2
- Jonathan W Newbury3
- Alan J Crockett1
- 1 Discipline of General Practice, University of Adelaide, Adelaide, SA.
- 2 School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA.
- 3 Spencer Gulf Rural Health School, University of South Australia and University of Adelaide, Port Lincoln, SA.
Simon Vanlint received a 2007 seeding grant through the Primary Health Care Research Evaluation and Development Program. Significant support with statistical and data analysis was provided by Fiona Mensah and Kara Cashman, Discipline of Public Health, University of Adelaide.
Howard Morris has spoken at meetings sponsored by Roche Diagnostics and Abbott Diagnostics; all honoraria were paid to his institution.
- 1. Matsuoka LY, Wortsman J, Haddad JG, et al. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol 1991; 127: 536-538.
- 2. Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 1998; 67: 1232-1236.
- 3. Rockell J, Skeaff CM, Williams SM, Green TJ. Association between quantitative measures of skin color and plasma 25-hydroxyvitamin D. Osteoporos Int 2008; 19: 1639-1642.
- 4. Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev 1986; 7: 434-438.
- 5. Moan J, Porojnicu AC, Robsahm TE, et al. Solar radiation, vitamin D and survival rate of colon cancer in Norway. J Photochem Photobiol B 2005; 78: 189-193.
- 6. Black P, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005; 128: 3792-3798.
- 7. Boucher B. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome “X”? Brit J Nutr 1998; 79: 315-327.
- 8. Hyppönen E, Läärä E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001; 358: 1500-1503.
- 9. Grant W, Garland C. Evidence supporting the role of vitamin D in reducing the risk of cancer. J Intern Med 2002; 252: 178-180.
- 10. Benson J, Wilson A, Stocks N, Moulding N. Muscle pain as an indicator of vitamin D deficiency in an urban Australian Aboriginal population. Med J Aust 2006; 185: 76-77. <MJA full text>
- 11. Scragg R, Sowers M-F, Bell C. Serum 25-hydroxyvitamin D, diabetes and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004; 27: 2813-2818.
- 12. Rockell JE, Green TJ, Skeaff CM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 years. J Nutr 2005; 135: 2602-2608.
- 13. Leslie WD, Krahn J, Weiler HA, et al. Vitamin D insufficiency in Canadian Aboriginal women: the First Nations Bone Health Study [abstract]. J Bone Miner Res 2004; 19 (1 Suppl): S423.
- 14. Morris HA, Morrison GW, Burr M, et al. Vitamin D and femoral neck fractures in elderly South Australian women. Med J Aust 1984; 140: 519-521.
- 15. van der Mei IA, Ponsonby AL, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect 2007; 115: 1132-1139.
- 16. Nowson C, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust 2002; 177: 149-152. <MJA full text>
- 17. Benjamin A, Moriakova A, Akhter N, et al. Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int 2009; 20: 1795-1803.
- 18. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002; 76: 187-192.
- 19. Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 2005; 90: 4119-4123.
- 20. Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British Birth Cohort: the role of obesity. Diabetes Care 2006; 29: 2244-2246.
- 21. Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur J Endocrinol 2004; 151: 167-172.
- 22. Jesudason D, Need AG, Horowitz M, et al. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 2002; 31: 626-630.
- 23. Aloia JF, Talwar SA, Pollack S, et al. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 2006; 84: 602-609.
- 24. Von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient — a randomised, placebo-controlled trial. Br J Nutr 2010; 103: 549-555.
- 25. Sabherwal S, Bravis V, Devendra D. Effect of oral vitamin D and calcium replacement on glycaemic control in South Asian patients with type 2 diabetes. Int J Clin Pract 2010; 64: 1084-1089.
- 26. Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr 1999; 53: 920-926.
- 27. Wack JT, Rodin J. Smoking and its effects on body weight and the systems of caloric regulation. Am J Clin Nutr 1982; 35: 366-380.
- 28. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009; 339: b3692.
- 29. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266-281.





Abstract
Objective: To investigate the adequacy of vitamin D status in a South Australian Aboriginal population, and to examine the relationship between serum 25-hydroxyvitamin D (25-OHD) levels and biochemical variables of calcium and bone mineral homeostasis, as well as other factors which may influence vitamin D synthesis, storage and metabolism.
Design, setting and participants: A single-visit, observational study of 58 adults from two Aboriginal community-controlled health services in Adelaide and Yalata, South Australia. Participants were recruited between May 2008 and December 2009.
Main outcome measures: Serum levels of 25-OHD, parathyroid hormone (PTH), fasting glucose and fasting C-terminal telopeptides of type I collagen (β-CTx).
Results: Serum 25-OHD levels showed clear seasonal variation, being higher in summer (P < 0.001). The overall mean level was 56.8 nmol/L (SD, 22.1), which is below the recommended target level of 60 nmol/L. Serum 25-OHD levels correlated significantly with β-CTx (P = 0.03), but not with age, body mass index (BMI), PTH levels or levels of fasting glucose. A significant association was found between BMI and PTH levels (P = 0.001). A significant inverse association between serum 25-OHD levels and BMI, observed in other studies, was not found in our study.
Conclusions: Vitamin D insufficiency is highly prevalent in this population of adult Aboriginal Australians, with low mean values found in all seasons other than summer.