In the absence of evidence of infection with the hepatitis C virus (HCV), detecting the immunological disorder of mixed cryoglobulinaemia is a challenge. Only after extensive investigation did we suspect that our patient’s recurrent acute dyspnoea, lower limb paraesthesia and renal impairment with active urinary sediment were attributable to the rare phenomenon of non-HCV-related mixed essential cryoglobulinaemia — our suspicion was confirmed by significant serum levels of cryoglobulins. (MJA 2010; 194: 142-144)
In October 2008, an 84-year-old man with increasing dyspnoea on exertion for 1 week was referred for hospital admission by his general practitioner. His medical history included rheumatic heart disease leading to aortic valve replacement (AVR) in 1973. In 2001, he underwent a redo AVR, a mitral valve replacement (non-mechanical) and coronary artery bypass grafting. He then required implantation of a permanent cardiac pacemaker for complete heart block. He had chronic kidney disease (Stage 3) of unknown cause and mild, well controlled asthma. He was a lifelong non-smoker and occasionally drank alcohol.
At presentation, he was afebrile and haemodynamically stable, with a respiratory rate of 20 breaths per minute and oximetry of 95 per cent on room air. There were no signs of fluid overload or peripheral stigmata of infective endocarditis. Chest auscultation revealed bibasal crackles, and loud first and second heart sounds in keeping with previous valve surgery. Mild stasis eczema was the only notable skin lesion. He reported experiencing, for the past few months, lower limb paraesthesia and sicca-like symptoms. His daily medications included perindopril 2.5 mg, digoxin 125 μg, frusemide 40 mg and warfarin at varying doses. For asthma, he was prescribed a daily inhalation of fluticasone 250 μg, with salmeterol 25 μg, and inhalations of salbutamol 100 μg as needed. Results of blood, urine and sputum tests done at presentation are shown in Box 1; results showed an inflammatory response and renal impairment with active urinary sediment (repeat urinary analysis a week after presentation also revealed active urinary sediment). An electrocardiogram showed no arrhythmias.
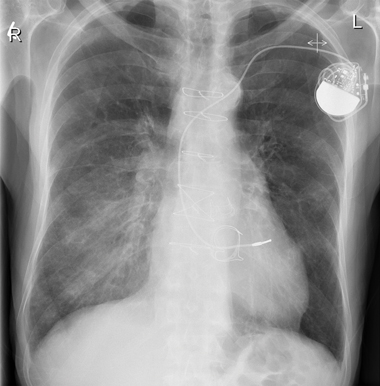
Chest x-ray at presentation showed air bronchograms at the right lower zone (Box 2). Our patient was admitted and treated for right lower lobe pneumonia with intravenous antibiotics. He was also prescribed high-dose oral steroids, with gradual tapering of doses. A computed tomography scan showed probable right lower chest infection.
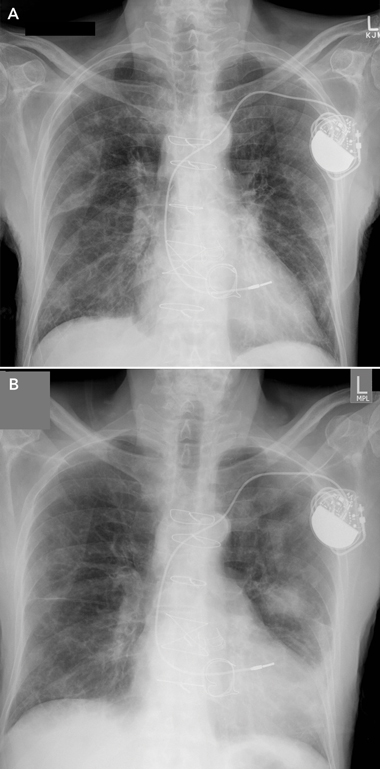
During the early weeks of his hospitalisation, the patient had further episodes of acute dyspnoea, and repeat chest x-rays showed bilateral migratory opacities (Box 3). Acute episodes of dyspnoea were treated with high-dose oral steroids and intravenous antibiotics; a regular regimen of low-dose steroids was maintained between episodes. During each acute episode, his biochemistry results showed an inflammatory response, with varying elevated serum levels of C-reactive protein (CRP): 243 mg/L; 332 mg/L; 73 mg/L; 16 mg/L; 148 mg/L; and 90 mg/L (reference range [RR], < 5.0 mg/L). With treatment, the CRP level fell to 5.3 mg/L. Repeated blood and urine cultures produced no growth.
A transthoracic echocardiogram revealed a normal left ventricular ejection fraction and no regurgitation from the prosthetic valves, and a transoesophageal echocardiogram showed no valvular vegetations. The serum level of B-type natriuretic peptide was 246 ng/L (RR, < 100 ng/L), making acute left ventricle failure unlikely in view of the patient’s renal impairment. No respiratory viruses were detected in nasopharyngeal aspirate. The patient’s serological tests were negative for hepatitis B virus, hepatitis C virus (HCV), HIV, cytomegalovirus, mycoplasma, Chlamydia, Legionella pneumophila, Legionella longbeachae, Brucella, Histoplasma, and Toxoplasma. The result of the QuantiFERON-TB Gold assay (Celeste) was indeterminate.
Serum electrophoresis was negative and no Bence–Jones protein was detected in urine. Liver ultrasound showed no evidence of chronic liver disease. Screening for autoimmune discrepancies was significant but inconclusive — test results for serum levels of rheumatoid factor (RF) and antinuclear antibody were positive but other antibody test results were negative or within reference ranges (Box 4).
On further investigation (5 weeks after admission), the patient’s serum levels of complement components C3 and C4 were 0.77 g/L (RR, 0.70–1.60 g/L) and < 0.02 g/L (RR, 0.10–0.30 g/L), respectively; repeated test results were C3, 0.74 g/L and C4, < 0.02 g/L. Such a result — low C4 with normal C3 levels — may be due to cryoglobulinaema, C4 null alleles, hereditary angioedema or activation of the classical complement pathway. Types and levels of cryoglobulins found in the patient’s serum were: monoclonal IgM/kappa, 0.4 g/L and polyclonal IgG, 0.1 g/L. HCV RNA was not detected.
On the basis of the presence of cryoglobulins in serum and supported by evidence of lung and inflammatory involvement, a diagnosis of mixed essential cryoglobulinaemia (Type II cryoglobulinaemia) was made. The patient was reviewed by an immunologist, who agreed with the diagnosis. To exclude lymphoma, particularly B-cell lymphoma which is the most frequent malignant complication of mixed cryoglobulinaemia, a bone marrow biopsy was done; no significant population of B-cell biomarkers (CD19+/CD20+ cells) was found, nor any other suggestion of lymphoma. Despite the investigative effort, we did not identify a cause for our patient’s cryoglobulinaemia. He gave informed consent for all investigations.
Cryoglobulins are single or mixed immunoglobulins that, in serum and other body fluids, undergo reversible precipitation at low temperatures (below 37°C in serum). According to the Brouet classification, cryoglobulinaemia is grouped into three types based on the composition of the detected cryoglobulins. Type I cryoglobulinaemia, or simple cryoglobulinaemia, is the result of a monoclonal immunoglobulin, which rarely has RF activity and is not known to activate complement in vitro. The cryoglobulins of Types II and III cryoglobulinaemia (mixed cryoglobulinaemia) contain RF, which forms complexes with the “fragment crystallisable” (Fc) portion of polyclonal IgG. The actual RF may be monoclonal immunoglobulin (in Type II cryoglobulinaemia) or polyclonal immunoglobulin (in Type III cryoglobulinaemia). Types II and III cryoglobulinaemia represent 80% of all occurrences of the disease. HCV is associated with most cases of mixed cryoglobulinaemia, with the prevalence of anti-HCV antibodies or HCV RNA ranging from 70% to almost 100%.1 The clinical manifestations of cryoglobulinaemia are generally caused by the inflammatory effects of circulating immune complexes on multiple organs.
The criteria for diagnosing mixed cryoglobulinaemia are: detection in serum of mixed cryoglobulins, along with purpura and leukocytoclastic vasculitis; or detection in serum of mixed cryoglobulins, along with peripheral neuropathy, membranoproliferative glomerulonephritis, chronic hepatitis or skin rashes.1 Signs and symptoms that our patient showed fulfilled the latter set of criteria. Although our patient had no skin lesion typical of the disease (and suitable for biopsy), nor evidence of liver disease, he had lower limb paraesthesia and tested positive for renal impairment and active urinary sediment. The success of steroid treatment indicated an inflammatory component to the problem. Furthermore, lung involvement is reported to be quite frequent in mixed essential cryoglobulinaemia,2 and migratory lung lesions have been associated with the disease.3 What is notable about this case is the lack of evidence of HCV infection. To the extent that we are able to establish (two PubMed searches of English-language articles were undertaken), it is the first reported case of mixed essential cryoglobulinaemia with migratory lung lesions and unrelated to HCV infection.
1 Results of patient’s blood, urine and sputum investigations at presentation
Test |
Result |
||||||||||||||
Blood |
|
||||||||||||||
Haemoglobin |
110 g/dL (RR, 135–180 g/dL) |
||||||||||||||
White cell count |
9.2 x 109 cells/L (RR, 4.0–11.0 x 109 cells/L) |
||||||||||||||
Neutrophils |
6.5 x 109 cells/L (RR, 2.0–8.0 x 109 cells/L) |
||||||||||||||
Platelets |
174 x 109 cells/L (RR, 140–400 x 109 cells/L) |
||||||||||||||
C-reactive protein |
243 mg/L (RR, < 5.0 mg/L) |
||||||||||||||
Sodium |
138 mmol/L (RR, 135–145 mmol/L) |
||||||||||||||
Potassium |
4.8 mmol/L (RR, 3.5–5.1 mmol/L) |
||||||||||||||
Urea |
13.3 mmol/L (RR, 2.9–8.2 mmol/L) |
||||||||||||||
Creatinine |
160 μmol/L (RR, 64–108 μmol/L) |
||||||||||||||
Cultures (three sets) |
No growth after 1 week incubation |
||||||||||||||
Urine |
|
||||||||||||||
Microscopic analysis |
Active sediment* |
||||||||||||||
Dipstick |
Moderate blood, 2 + leukocytes, trace protein |
||||||||||||||
Culture |
No growth after 1 week incubation |
||||||||||||||
Sputum |
|
||||||||||||||
Culture |
Normal respiratory flora |
||||||||||||||
RR = reference range. * Active urinary sediment is sediment found in a centrifuged urine sample and showing red cells, red cell casts and at times white cells and white cell casts; it indicates active kidney inflammatory disease such as glomerulonephritis, interstitial nephritis or vasculitis. |
|||||||||||||||
2 Patient’s chest x-ray at presentation showing air bronchograms at right lower zone (2 October 2008)
3 Patient’s chest x-rays several weeks after presentation showing bilateral migratory opacities (A, 16 November 2008; B, 24 November 2008)
4 Results of patient’s autoimmunity screening tests at presentation and 4 weeks after presentation
Test |
Initial result |
Result at 4 weeks |
|||||||||||||
Rheumatoid factor (RR, < 20 IU/mL) |
51 IU/mL |
< 20 IU/mL |
|||||||||||||
Anticyclic citrullinated peptide antibody (anti-CCP) |
< 6 U/mL |
— |
|||||||||||||
Antinuclear antibody (ANA) (RR, < 40 titre) |
> 2560 titre |
640 (homogenous) titre |
|||||||||||||
Anti-double-stranded DNA antibodies |
negative |
— |
|||||||||||||
Anti-Sjögren’s syndrome A / anti-Sjögren’s syndrome B antibodies (anti SSA/SSB antibodies) |
negative |
— |
|||||||||||||
Direct Coombs test |
negative |
— |
|||||||||||||
Antithyroid peroxidise antibodies (RR, < 50 IU/mL) |
1 IU/mL |
— |
|||||||||||||
Antithyroglobulin antibodies (RR, < 100 IU/mL) |
7 IU/mL |
— |
|||||||||||||
Antitissue transglutaminase antibody (anti-TTG [IgA]) (RR, < 5 U/mL) |
0 U/mL |
— |
|||||||||||||
RR = reference range |
|||||||||||||||
- 1. Ferri C. Mixed cryoglobulinaemia. Orphanet J Rare Dis 2008; 3: 25. http://www.ojrd.com/content/3/1/25 (accessed Nov 2010).
- 2. Bombardier S, Paoletti P, Ferri C, et al. Lung involvement in essential mixed cryoglobulinemia [abstract]. Am J Med 1979; 66: 748. http://www.amjmed.com/article/0002-9343(79)91112-4/abstract (accessed Nov 2010).
- 3. Zackrison LH, Katz P. Bronciolitis obliterans organizing pneumonia associated with essential mixed cryoglobulinemia. Arthritis Rheum 1993, 36: 1627-1630.





None identified.