The ABCD2 (age, blood pressure, clinical features, duration and diabetes) score was developed as a simple clinical tool to predict which patients may be at highest risk of early stroke following transient ischaemic attack (TIA),1 and is determined by assigning points for the presence of specific features (Box 1). Several management guidelines for TIA recommend its use to guide clinical management or hospital admission policy.2-5 Previous Australian guidelines have recommended that patients identified as being at low risk (ABCD2 score, ≤ 4) may be managed in the community.5
Recently, several reports have been published involving non-Australian patient samples that question the predictive value of the ABCD2 score.6,7 There are currently no published reports of its predictive value for early stroke risk in Australian settings, limiting the confidence with which it can be applied. In this study, we examine the predictive value of the ABCD2 score for stroke occurring within 90 days of an index TIA in a large Australian tertiary-hospital cohort.
We studied consecutive patients with suspected TIA referred by the emergency department (ED) to the acute stroke unit (in accordance with the TIA pathway) at Monash Medical Centre, an urban tertiary referral hospital in Melbourne, Victoria, between 1 June 2004 and 30 November 2007. The study was approved by the Southern Health Hospital Research Ethics Committee.
All patients were initially evaluated by an ED physician and TIA diagnoses were confirmed by an expert stroke physician. TIA was defined as “acute loss of focal cerebral or monocular function with symptoms lasting less than 24 hours and which is thought to be due to inadequate cerebral or ocular blood supply as a result of arterial thrombosis or embolism”.8 Patients whose symptoms lasted longer than 24 hours were excluded from the study. Patients were treated according to an accelerated ED-based protocol, supervised by stroke unit physicians, which was designed to facilitate rapid investigations and urgent commencement of antiplatelet or anticoagulation therapy after brain imaging, before discharge from ED.
Data for individual ABCD2 components were collected prospectively during ED assessment according to published criteria (Box 1).1 Blood pressure was taken from the first reading in the ED. Other clinical features and duration of symptoms were determined directly from patients or relatives. The ABCD2 scores were not used to guide clinical management and were calculated independently for each patient. Patients were then classified as being at either high or low early risk of stroke according to a range of prespecified cut-off values used in the original validation study1 and current Australian recommendations.5
Stroke occurring within 2, 7 and 90 days was recorded using the World Health Organization definition of stroke as “rapidly developed clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than of vascular origin”.9 Outcome was determined from multiple sources, including specialist clinic reviews, outpatient and hospital records, and a validated telephone interview10 by independent personnel who were blinded to ABCD2 data or scores. Results of carotid ultrasound and electrocardiography were also reviewed to determine the mechanism of the stroke.
Descriptive statistics for patient demographic characteristics and vascular risk profile were calculated. The proportions of patients suffering stroke within 2, 7 and 90 days were calculated. The area under the curve (AUC) for the receiver operating characteristic (ROC) curve was determined.11 An AUC is obtained after plotting the true-positive rate (sensitivity) versus the false-positive rate (1 − specificity), and summarises the accuracy of the ABCD2 score over the entire range of possible ABCD2 values. In this case, the AUC demonstrates the capacity of the score to discriminate between high and low risk of stroke. An AUC of 1.0 indicates perfect accuracy (ie, that a patient randomly selected from the outcome group [stroke] will have a higher ABCD2 score than a patient randomly selected from the outcome-free [stroke-free] group 100% of the time), while an AUC of 0.5 indicates performance no better than chance. To evaluate clinical predictive utility, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive likelihood ratio (PLR) for stroke were obtained for several ABCD2 cut-off scores. Stata, version 8.0 (StataCorp, College Station, Tex, USA) was used for analyses.
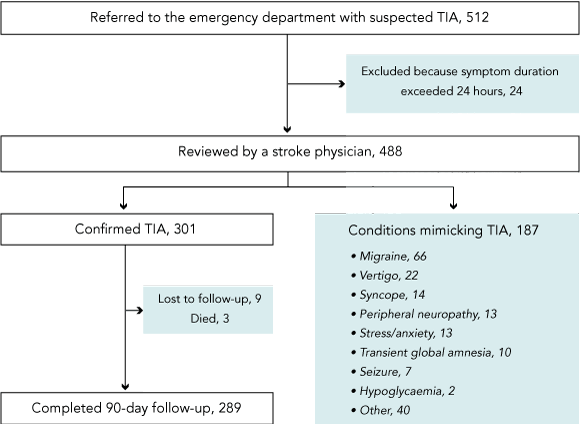
Overall, 512 patients were referred with suspected TIA. Of these, 24 were excluded because their symptoms lasted more than 24 hours, consistent with a diagnosis of minor stroke and not TIA. Of the 488 remaining patients, 301 (61.7%) had a confirmed TIA (Box 2). Characteristics of the patients with confirmed TIA are presented in Box 3, stratified by ABCD2 cut-off scores. At presentation, 56.4% of patients with confirmed TIA were receiving either antiplatelet or anticoagulant medication; this had increased to 98.4% at clinic review. Atrial fibrillation (AF) was present in 14.6% (44/301) and 10% (30/301) had ipsilateral carotid stenosis ≥ 70%. Seventeen patients underwent urgent carotid intervention.
Complete follow-up was achieved in 96.0% of patients with confirmed TIA (289/301). There were no stroke-related deaths and no differences in vascular profile between those completing and those lost to follow-up, excepting that diabetes mellitus was absent among patients lost to follow-up (data not shown). The proportion of patients who had a stroke after confirmed TIA was 1.37% (4/292; 95% CI, 0.37–3.47%) at both 2 and 7 days, and 2.42% (7/289; 95% CI, 0.98–4.93) at 90 days. Details of patients with stroke at 90 days are shown in Box 4.
Box 5 shows that the AUC for stroke after confirmed TIA was 0.80 (95% CI, 0.68–0.91) at 2 days and 0.62 (95% CI, 0.40–0.83) at 90 days. The performance of different ABCD2 cut-off scores is shown in Box 6. For a cut-off ≥ 4, the ABCD2 score had poor specificity, PPV and PLR with only modest specificity at cut-off ≥ 5. These results were similar at all prespecified cut-off scores and when conditions mimicking TIA were included.
Applying the cut-off score of ≤ 4 for low risk5 inappropriately classified 11.6% of patients (35/301) as being at low risk — 21 patients with AF and 16 with ipsilateral carotid stenosis of ≥ 70% (two patients had both). This represents 53% of patients with ≥ 70% ipsilateral carotid stenosis (16/30) and 48% of patients with AF (21/44).
We have shown that the ABCD2 score has poor clinical predictive value for stroke within 90 days of TIA in an Australian tertiary hospital setting. The use of cut-off scores previously recommended in Australia,5 also led to almost half of patients with ipsilateral carotid stenosis and AF being misclassified as being at low risk of stroke.12 Our findings add to the uncertainty about the current validity of the ABCD2 score.6,7,13
The strengths of our study include the large sample of consecutive patients, high follow-up rates, standardised definitions, prospective ABCD2 data, independence of clinical management from the ABCD2 score, and independent recording of outcomes. Our results may thus be applicable to other tertiary Australian hospital settings with developed TIA pathways. The few patients lost to follow-up had similar vascular profiles to those with complete follow-up, making a higher stroke rate unlikely in this group. A limitation of our study is the tertiary hospital-based nature of our sample, leaving open the question of whether it is a useful tool in primary care settings.
The AUC of 0.80 for stroke after confirmed TIA at 2 days (Box 5) indicates that a patient randomly selected from the group who had stroke will have a higher ABCD2 score than a patient randomly selected from the stroke-free group 80% of the time, leaving considerable room for misclassification. The confidence interval for the AUC at 90 days of 0.40–0.83 indicates the ABCD2 score accuracy at 90 days may be no better than chance.14 However, the AUC is a pretest estimate of accuracy, and does not assist in clinical prediction in an individual patient. For individual prediction of high risk, a high specificity, high PPV and high PLR (greater than 10) are desirable,11 none of which were demonstrated in our sample.
Our findings are consistent with those of some previous reports,6,7,13 but comparisons with others1,15 is difficult as they only present AUCs. If the false-positive rate for high risk is large as shown in our study, then admitting a patient based only on the ABCD2 score may lead to injudicious use of hospital beds in an already stretched environment. Admission policy may be better dictated by suspected stroke mechanism or the presence of crescendo TIA, and should be guided by locally available resources.
In spite of high NPV values in our study, there was considerable misclassification of patients with high-risk stroke mechanisms (carotid stenosis and AF), findings similar to those of a previous study.7 This could have serious implications for patient safety if clinicians delay urgent investigations based solely on the ABCD2 score. It is more crucial to have systems in place that may expedite the urgent detection and treatment of AF and carotid stenosis. Indeed, the authors who originally developed the ABCD2 score caution that it should not supersede expert neurological judgement in the clinical management of TIA.1
As has been found in other studies in settings where aggressive secondary prevention is practised,13,16,17 we report a low stroke rate following TIA. The original ABCD2 derivation18,19 and validation cohorts1 were established in a setting of suboptimal secondary stroke prevention, reflected in the relatively high stroke rate reported (around 10%). In one cohort, only 57% of patients received aspirin at any time during the follow-up period.19 Applying a theoretical 10% stroke rate to our sample (an estimated 29 patients), and assuming all extra patients who had strokes were correctly classified as high risk (ABCD2 score ≥ 5), the estimated PPV would still be extremely low (around 24%). We also report low PLR estimates (which are independent of prevalence20) for all prespecified cut-offs of the ABCD2 score, confirming its poor predictive value in patients treated aggressively. More precise estimates may, however, be obtained with larger cohorts.
In summary, the ABCD2 score, if used alone, has poor predictive value for early stroke after TIA in patients presenting to an Australian tertiary hospital. There may be no substitute for careful and rapid clinical evaluation and individualising management based on the underlying stroke mechanism.
1 The ABCD2 score1
ABCD2 criteria |
Points |
||||||||||||||
Age ≥ 60 years |
1 |
||||||||||||||
Blood pressure ≥ 140/90 mmHg |
1 |
||||||||||||||
Clinical features of TIA |
|
||||||||||||||
Weakness* |
2 |
||||||||||||||
Speech impairment† |
1 |
||||||||||||||
Duration of symptoms |
|
||||||||||||||
≥ 60 min |
2 |
||||||||||||||
10–59 min |
1 |
||||||||||||||
Diabetes mellitus |
1 |
||||||||||||||
Total ABCD2 score‡ |
0–7 |
||||||||||||||
TIA = transient ischaemic attack. * Defined unilateral weakness. † In the absence of unilateral weakness. ‡ Stroke risk at 2 days:1 score 0–3, 1.0%; score 4–5, 4.1%; score 6–7, 8.1%. |
|||||||||||||||
3 Characteristics of 301 patients who had transient ischaemic attack by three different ABCD2 cut-off scores
|
Cut-off ≥ 4 |
Cut-off ≥ 5 |
Cut-off ≥ 6 |
||||||||||||
ABCD2 score |
0–3 |
≥ 4 |
0–4 |
≥ 5 |
0–5 |
≥ 6 |
|||||||||
No. of patients |
87 |
214 |
173 |
128 |
246 |
55 |
|||||||||
61.1 ± 12.7 |
70.3 ± 12.3 |
64.1 ± 13.1 |
72.5 ± 11.4 |
66.2 ± 13.4 |
74.3 ± 9.4 |
||||||||||
Male |
50 (57.5%) |
125 (58.4%) |
103 (59.5%) |
72 (56.3%) |
144 (58.5%) |
31 (56.4%) |
|||||||||
Hypertension |
48 (55.2%) |
155 (72.4%) |
105 (60.7%) |
98 (76.6%) |
157 (63.8%) |
46 (83.6%) |
|||||||||
Hyperlipidaemia |
50 (57.5%) |
129 (60.3%) |
100 (57.8%) |
79 (61.7%) |
146 (59.3%) |
33 (60.0%) |
|||||||||
Diabetes mellitus |
7 (8.0%) |
73 (34.1%) |
24 (13.9%) |
56 (43.8%) |
49 (19.9%) |
31 (56.4%) |
|||||||||
Ever smoked |
29 (33.3%) |
57 (26.6%) |
54 (31.2%) |
32 (25.0%) |
68 (27.6%) |
18 (32.7%) |
|||||||||
Atrial fibrillation |
7 (8.0%) |
37 (17.3%) |
21 (12.1%) |
23 (18.0%) |
36 (14.6%) |
8 (14.5%) |
|||||||||
Ipsilateral carotid stenosis ≥ 70% |
9 (10.3%) |
21 (9.8%) |
16 (9.2%) |
14 (10.9%) |
22 (8.9%) |
8 (14.5%) |
|||||||||
4 Details of patients experiencing stroke within 90 days of transient ischaemic attack
Patient |
Sex |
ABCD2 score |
Days to stroke |
Stroke mechanism |
|||||||||||
1 |
Female |
6 |
0 |
Cryptogenic |
|||||||||||
2 |
Male |
6 |
0 |
Carotid stenosis |
|||||||||||
3 |
Male |
5 |
1 |
Lacunar |
|||||||||||
4 |
Male |
5 |
2 |
Carotid stenosis |
|||||||||||
5* |
Male |
3 |
30 |
Carotid stenosis |
|||||||||||
6† |
Female |
5 |
60 |
Atrial fibrillation and cardioembolism |
|||||||||||
7 |
Male |
3 |
86 |
Cryptogenic |
|||||||||||
* Stroke occurred as complication of digital subtraction angiography. † Stroke occurred in a setting of atrial fibrillation and suboptimal warfarin dosing. |
|||||||||||||||
5 Receiver operating characteristic (ROC) analysis* of the ABCD2 score in patients with transient ischaemic attack for stroke outcome at 2 days and 90 days
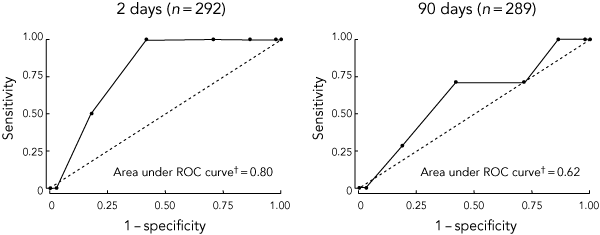
* ROC analysis plots the true-positive rate (sensitivity) versus the false-positive rate (1 − specificity). The eight data points represent the true-positive versus false-positive rates for the ABCD2 scores 0–7. † Indicates the overall capacity of the ABCD2 score to discriminate between high and low risk of stroke, with a value of 1.0 indicating perfect accuracy, and a value of 0.5 indicating performance no better than chance.
6 Performance of varying ABCD2 cut-off scores in predicting stroke after transient ischaemic attack
Group* |
ABCD2 cut-off score |
Strokes |
Patients followed up |
Sensitivity† |
Specificity† |
Positive predictive value† |
Negative predictive value† |
Positive likelihood ratio† |
|||||||
Stroke by 2 days |
|
|
|
|
|
|
|
|
|||||||
|
≥ 4 |
4 |
292 |
1.00 (0.40–1.00) |
0.29 (0.24–0.35) |
0.02 (0.01–0.05) |
1.00 (0.96–1.00) |
1.41 (1.31–1.52) |
|||||||
|
≥ 5 |
4 |
292 |
1.00 (0.40–1.00) |
0.58 (0.52–0.64) |
0.03 (0.01–0.08) |
1.00 (0.98–1.00) |
2.40 (2.09–2.75) |
|||||||
|
≥ 6 |
2 |
292 |
0.50 (0.07–0.93) |
0.82 (0.77–0.86) |
0.04 (0.01–0.13) |
0.99 (0.97–1.00) |
2.77 (1.01–7.61) |
|||||||
Stroke by 90 days |
|
|
|
|
|
|
|
|
|||||||
|
≥ 4 |
5 |
289 |
0.71 (0.29–0.96) |
0.29 (0.24–0.34) |
0.02 (0.01–0.06) |
0.98 (0.92–1.00) |
1.00 (0.62–1.61) |
|||||||
|
≥ 5 |
5 |
289 |
0.71 (0.29–0.96) |
0.58 (0.52–0.64) |
0.04 (0.01–0.09) |
0.99 (0.96–1.00) |
1.71 (1.05–2.78) |
|||||||
|
≥ 6 |
2 |
289 |
0.29 (0.04–0.71) |
0.82 (0.77–0.86) |
0.04 (0.00–0.13) |
0.98 (0.95–0.99) |
1.55 (0.47–5.13) |
|||||||
* Values for stroke at 7 days are not shown because they are exactly the same as those for stroke at 2 days. † Values in parentheses are 95% confidence intervals. |
|||||||||||||||
- Lauren M Sanders1,2
- Velandai K Srikanth1,2
- Helen Psihogios3
- Kitty K Wong1,2
- David Ramsay2
- Thanh G Phan1,2
- 1 Department of Medicine, Southern Clinical School, Monash University, Melbourne, VIC.
- 2 Department of Neurology, Southern Health, Melbourne, VIC.
- 3 Department of Emergency Medicine, Southern Health, Melbourne, VIC.
None identified.
- 1. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369: 283-292.
- 2. National Institute for Health and Clinical Excellence. Stroke. Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). http://www.nice.org.uk/CG068 (accessed Dec 2010).
- 3. National Collaborating Centre for Chronic Conditions. Stroke: National clinical guideline for diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). London: Royal College of Physicians, 2008. http://www.nice.org.uk/nicemedia/live/12018/41363/41363.pdf (accessed Dec 2010).
- 4. Institute for Clinical Systems Improvement. Health care guidelines. Stroke, ischemic, diagnosis and initial treatment of (guideline). http://www.icsi.org/guidelines_and_more/gl_os_prot/cardiovascular/stroke/stroke__ischemic__diagnosis_and_initial_treatment_of_.html (accessed Dec 2010).
- 5. National Stroke Foundation. Clinical guidelines for acute stroke management. National Stroke Foundation 2007. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/cp109.pdf (accessed Sep 2009).
- 6. Asimos AW, Johnson AM, Rosamond WD, et al. A multicenter evaluation of the ABCD2 score’s accuracy for predicting early ischemic stroke in admitted patients with transient ischemic attack. Ann Emerg Med 2009; 55: 201-210.
- 7. Amarenco P, Labreuche J, Lavallee PC, et al. Does ABCD2 score below 4 allow more time to evaluate patients with a transient ischemic attack? Stroke 2009; 40: 3091-3095.
- 8. Hankey G, Warlow C. Evolution of the concepts of TIAs. In: Hankey G. Transient ischaemic attacks of the brain and eye. London: WB Saunders, 1994: 1-9.
- 9. Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980; 58: 113-130.
- 10. Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke 2000; 31: 1076-1080.
- 11. Fletcher RH, Fletcher SW, Wagner EH. Clinical epidemiology: the essentials. 2nd ed. Baltimore: Williams & Wilkins, 1988.
- 12. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004; 62: 569-573.
- 13. Ghia D, Thomas P, Epstein E, et al. Early stroke and ABCD2 scores in a large Australian transient ischaemic attack cohort [abstract]. Int J Stroke 2009; 4: 9-10.
- 14. Marzban C. A comment on the ROC curve and the area under it as performance measures. Weather and Forecasting 2004; 19: 1106-1114.
- 15. Chandratheva A, Geraghty OC, Luengo-Fernandez R, et al. ABCD2 score predicts severity rather than risk of early recurrent events after transient ischemic attack. Stroke 2010; 41: 851-856.
- 16. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432-1442.
- 17. Lavallee PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007; 6: 953-960.
- 18. Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284: 2901-2906.
- 19. Dennis M, Bamford J, Sandercock P, et al. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke 1990; 21: 848-853.
- 20. Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004; 329: 168-169.





Abstract
Objective: To determine the predictive value of the ABCD2 score for early risk of stroke in Australian patients who have had transient ischaemic attack (TIA).
Design, participants and setting: Cohort study of 512 consecutive patients with suspected TIA referred by the emergency department to the acute stroke unit (in accordance with the TIA pathway) of an urban tertiary hospital in Melbourne, Victoria, between 1 June 2004 and 30 November 2007.
Main outcome measures: Overall accuracy, estimated by the area under the curve (AUC) of receiver operating characteristic plots (of true positive rate v false positive rate), and sensitivity, specificity, predictive values and likelihood ratios at prespecified cut-off ABCD2 scores for stroke within 2, 7 and 90 days.
Results: 24 patients were excluded because their symptoms lasted more than 24 hours. All included patients were reviewed by a stroke physician; TIA was confirmed in 301/488 (61.7%). Most (289/301; 96.0%) had complete follow-up. Stroke occurred in 4/292 patients (1.37%; 95% CI, 0.37%–3.47%) within 2 days and 7/289 (2.42%; 95% CI, 0.98%–4.93%) within 90 days; no patient had a stroke between 2 and 7 days. The AUCs for stroke in patients with confirmed TIA were 0.80 (95% CI, 0.68–0.91) and 0.62 (95% CI, 0.40–0.83) for stroke within 2 days and 90 days, respectively. At a cut-off of ≥ 5, the ABCD2 score had modest specificity for stroke within 2 days (0.58) and 90 days (0.58), but positive predictive values (2 days, 0.03; 90 days, 0.04) and positive likelihood ratios (2 days, 2.40; 90 days, 1.71) were both poor. The score performed similarly poorly at other prespecified cut-off scores.
Conclusions: Given its poor predictive value, the use of the ABCD2 score alone may not be dependable for guiding clinical treatment decisions or service organisation in an Australian tertiary setting. Validation in other Australian settings is recommended before it can be applied with confidence.