Cimicifuga racemosa, better known as black cohosh, has been widely used in Western cultures as a herbal treatment for relieving symptoms of menopause. It has previously been linked to cases of liver toxicity. We report a case of reversible complete heart block in a woman who had recently begun taking a herbal supplement containing black cohosh. We review the known side effect profile of black cohosh and its relationship to our case.
While undergoing cardiac monitoring in the emergency department, the patient experienced a further episode of syncope. Telemetry (Box 1, A) and an electrocardiogram (ECG) (Box 1, B) demonstrated complete heart block. An atropine bolus was administered and an isoprenaline infusion commenced. The initial ECG performed after commencement of isoprenaline demonstrated 2:1 heart block. The patient’s serum electrolyte levels were normal.
The only listed active ingredient of Remifemin is isopropanolic Cimicifuga racemosa root extract, also known as Actaea racemosa and most commonly known as black cohosh (BC). Remifemin contains the most thoroughly researched formulation of BC.1 BC was traditionally used by Native Americans of Canada and the eastern United States to treat malaria, impaired kidney function, sore throat, rheumatism, menstrual irregularities, and pain during childbirth.2 Recently, there has been interest in its use in the treatment of menopausal symptoms.
A recent systematic review identified over 72 studies of BC,3 but only 13 of these were clinical studies involving BC-only preparations published since an earlier review in 2003.4 Findings regarding adverse events were consistent with those of another earlier review, which had found that in more than 2800 patients, the rate of adverse events was about 5.4%, and over 97% of events were minor.3 Most adverse events identified by the more recent review were gastrointestinal symptoms and musculoskeletal and connective tissue disorders.3 Three recently published reviews have examined hepatotoxicity3,5,6 — the most commonly reported serious adverse event associated with BC. They described seven, 42 and 31 cases of hepatotoxicity, respectively, but all three concluded that, in general, data supporting definite causality are lacking. The US Pharmacopeia Dietary Supplements Information Expert Committee, the European Medicines Agency, and the Australian Therapeutic Goods Administration (TGA) recommend that preparations containing BC should carry a warning of possible hepatotoxicity.5-7 Other serious adverse events reported include anaphylaxis, cutaneous vasculitis and myotoxicity.8-10 Studies of BC for mutagenicity, teratogenicity and carcinogenicity have produced negative findings.11
The mechanism by which BC exerts its effects is uncertain. The rhizome of BC contains a number of biologically active constituents, including the triterpene glycosides actein, 27-deoxyactein and cimicifugoside, as well as long-chain fatty acids, resins, caffeic acids, isoferulic acids, phytosterin, fukinolic acid, salicylic acid, sugars and tannins.12 To date, over 50 compounds derived from BC have been described.13
Serotonergic effects not due to serotonin selective reuptake inhibition have been demonstrated with BC preparations.14,15 BC exhibits competitive binding to the 5-HT1A, 5-HT1D and 5-HT7 receptors14-16 and is a partial agonist at serotonin receptors.17 This is noteworthy, as studies show that activation of 5-HT1A receptors in the hypothalamus inhibit hypothalamus-mediated increases in heart rate and blood pressure.18,19
One study investigating the vasoactive effects of BC demonstrated that BC-derived cimicifugic acids inhibit noradrenaline-mediated contraction in rat aortas by inhibition of calcium influx.20 In their 1993 review,4 Borelli and colleagues described a 1935 study in which four glycosidic fractions obtained from the rhizome of BC were administered to dogs; the fraction insoluble in water was found to induce strong arterial hypotension, a decrease in cardiac contraction, and bradycardia to the point of death.
Based on the published pharmacology of the components of BC, it is difficult to provide a clear explanation as to how it mediates complete heart block. It is noteworthy that bradycardia is a widely listed side effect of BC in non-academic literature and that profound bradycardia has been documented in animal studies following administration of its extracts.4 Applying the Naranjo algorithm to this case shows that BC was probably responsible for the presentation of our patient (Box 2).21 Given the severity of the adverse reaction and the 2-week delay until its onset, reintroduction of BC while appropriately monitoring the patient to strengthen the argument for causality is not feasible.
1 Patient’s telemetry and electrocardiogram (ECG) traces
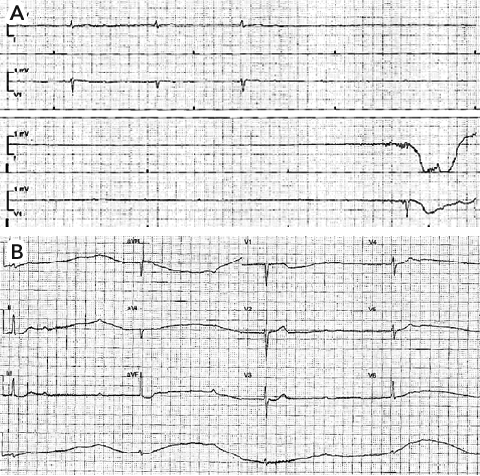 |
|
Cardiac monitoring telemetry trace (A) and ECG (B) showing complete heart block. |
- 1. Briese V, Stammwitz U, Friede M, Henneicke-von Zepelin HH. Black cohosh with or without St. John’s wort for symptom-specific climacteric treatment — results of a large-scale, controlled, observational study. Maturitas 2007; 57: 405-414.
- 2. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms (protocol). Cochrane Database Syst Rev 2008; (3): CD007244. doi: 10.1002/14651858.CD007244.
- 3. Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa): a systematic review of adverse events. Am J Obstet Gynecol 2008; 199: 455-466.
- 4. Borelli F, Izzo AA, Ernst E. Pharmacological effects of Cimicifuga racemosa. Life Sci 2003; 73: 1215-1229.
- 5. Mahady GB, Low Dog T, Barrett ML, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008; 15 (4 Pt 1): 628-638.
- 6. European Medicines Agency Committee on Herbal Medicinal Products. Annex 1: Assessment of case reports connected to herbal medicinal products containing Cimicifugae racemosae rhizoma (black cohosh, root). London: EMEA, 8 May 2007. http://www.emea.europa.eu/pdfs/human/hmpc/26925806en.pdf (accessed Sep 2009).
- 7. Therapeutic Goods Administration Adverse Drug Reactions Advisory Committee. Hepatotoxicity with black cohosh. Aust Adverse Drug React Bull 2006; 25 (2). http://www.tga.gov.au/adr/aadrb/aadr0604.htm#a1 (accessed Sep 2009).
- 8. Frempong W, Kiuru A, Ericsson J, Farah M. Cimicifuga racemosa L. Nutt. (black cohosh) and anaphylactic reactions, including face and oral oedema. Uppsala, Sweden: Uppsala Monitoring Centre. http://www.who-umc.org/graphics/6998.pdf (accessed Sep 2009).
- 9. Ingraffea A, Donohue K, Wilkel C, Falanga V. Cutaneous vasculitis in two patients taking an herbal supplement containing black cohosh. J Am Acad Dermatol 2007; 56 (5 Suppl): S124-S126.
- 10. Minciullo PL, Saija A, Patafi M, et al. Muscle damage induced by black cohosh (Cimicifuga racemosa). Phytomedicine 2006; 13: 115-118.
- 11. Pepping J. Black cohosh: Cimicifuga racemosa. Am J Health Syst Pharm 1999; 56: 1400-1402.
- 12. Mills S, Bone K. Principles and practice of phytotherapy: modern herbal medicine. Edinburgh: Churchill Livingstone, 2000.
- 13. Nuntanakorn P, Jiang B, Einbond LS, et al. Polyphenolic constituents of Actaea racemosa. J Nat Prod 2006; 69: 314-318.
- 14. Powell SL, Gödecke T, Nikolic D, et al. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem 2008; 56: 11718-11726.
- 15. Burdette JE, Liu J, Chen SN, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem 2003; 51: 5661-5670.
- 16. Gödecke T, Nikolic D, Lankin DC, et al. Phytochemistry of cimicifugic acids and associated bases in Cimicifuga racemosa root extracts. Phytochem Anal 2009; 20: 120-133.
- 17. Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 inhibitors from the black cohosh (Cimicifuga racemosa). Evid Based Complement Alternat Med 2005; 2: 223-226.
- 18. Horiuchi J, McDowall LM, Dampney RA. Role of 5-HT(1A) receptors in the lower brainstem on the cardiovascular response to dorsomedial hypothalamus activation. Auton Neurosci 2008; 142: 71-76.
- 19. Villela DC, da Silva LG Jr, Fontes MA. Activation of 5-HT receptors in the periaqueductal gray attenuates the tachycardia evoked from dorsomedial hypothalamus. Auton Neurosci 2009; 148: 36-43.
- 20. Noguchi M, Nagai M, Koeda M, et al. Vasoactive effects of cimicifugic acids C and D, and fukinolic acid in cimicifuga rhizome. Biol Pharm Bull 1998; 21: 1163-1168.
- 21. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-245.





None identified.