Ovarian cancer is the leading cause of death from gynaecological malignancies in Western countries and the fifth most common cancer causing death in women.1 Psychological morbidity following a diagnosis of cancer is well documented;2,3 up to 45% of patients with cancer experience clinically significant anxiety, and 25% experience clinically significant depression.4 Among the few small and unrepresentative studies focusing on ovarian cancer, rates of clinical anxiety of 22%–29%, and of clinical depression of 17%–21% have been reported.5 The impact of cancer on family members, in particular, spouses, is increasingly being recognised. Distress among caregivers is reported at 20%–30%, and as high as 50% in caregivers of patients with advanced disease or receiving palliative care.6 Some studies suggest that caregiver anxiety and depression is often more common and more severe than that of the patients.7
Untreated anxiety and depression is associated with poor symptom control, non-adherence to treatment recommendations, and increased time in hospital.8 Thus, it is critical to understand the antecedents of morbidity in order to provide appropriate services. Higher optimism in patients has been associated with better emotional wellbeing,9 while feelings of hopelessness and absence of a partner are associated with higher depression in patients, both in the short term,10 and long term.4,11 However, no studies have explored the specific antecedents of distress in patients with ovarian cancer and their caregivers.
Timely access to professional services might be expected to alleviate distress. Concerningly, patients report receiving less advice and information about psychosocial support than health-related information.12 The rate of participation in counselling or support groups among patients with cancer is reported at 14%–24%,13,14 and only about 25% of distressed patients receive treatment.15 Similarly, few caregivers seek out and receive additional psychosocial support.16
While patients report that they may have used services if they had known about them,12 availability and access to mental health and other support services may influence actual uptake, especially in rural and regional areas.17
In this study, our aims were to:
describe the prevalence of anxiety and depression in women with ovarian cancer and their caregivers, and compare with community norms;
compare levels of anxiety and depression in patients and caregivers; and
identify demographic, medical and psychological predictors of anxiety and depression in women with ovarian cancer and their caregivers.
Patients participating in the Quality of Life (QoL) substudy of the Australian Ovarian Cancer Study (AOCS) were recruited through the AOCS. The AOCS is a population-based study of women aged 18–79 years newly diagnosed with primary ovarian cancer, fallopian tube cancer or peritoneal cancer between 1 January 2002 and 30 June 2006.18 Women were recruited through major treatment centres and state-based cancer registries. The AOCS collected detailed ovarian cancer risk-factor data relating to the period ending 1 year before diagnosis, disease and initial treatment data, as well as ongoing treatment and clinical outcome data. The QoL substudy is investigating the role of psychosocial factors in predicting outcomes, collecting data by questionnaire at baseline and then every 3 to 6 months for up to 2 years. If more than one item on any questionnaire was missing, the participant was contacted to complete the items; missing psychosocial data are therefore minimal. In this article, we report data from baseline questionnaires only.
The primary outcomes, anxiety and depression, were assessed using the Hospital Anxiety and Depression Scale (HADS).19 This scale has two subscales which distinguish between anxiety and depression. Scores on each subscale delineate “normal” (score, 0–7), “subclinical” (score, 8–10), and “clinical” (score, 11–21) levels of anxiety and depression.
Predictor variables included those listed below.
Disease, treatment and symptom burden data: Data on date of diagnosis and surgical stage (according to the International Federation of Gynecology and Obstetrics [FIGO]) at diagnosis were obtained through the AOCS. Current treatment type (chemotherapy, radiotherapy) was collected by questionnaire. Symptom burden was assessed by the 12-item additional concerns subscale of the Functional Assessment of Cancer Therapy–Ovarian scale.20 Scores on this scale range from zero to 44, with higher scores indicating lower symptom burden.
Social support: This was assessed using the Duke University of North Carolina Functional Social Support Questionnaire.21 Higher scores indicate greater support.
Optimism: This was assessed using the Life Orientation Test–Revised, a widely used measure of dispositional optimism.22 Higher scores indicate higher optimism.
Data were analysed using SPSS, version 17 (SPSS Inc, Chicago, Ill, USA). χ2 tests were used to compare proportions of individuals whose scores indicated normal, subclinical or clinical anxiety and depression on the HADS subscales, compared with normative data23 (these were British, as no Australian normative HADS data are currently available), and other categorical variables. The Wilcoxon test was used to directly compare patient–caregiver pairs on raw anxiety and depression scores. To examine predictors, anxiety and depression data were recoded as either normal or subclinical/clinical, and analysed using binary logistic regression, including demographic, disease and psychosocial variables as covariates.
A total of 798 eligible AOCS patients (66%) completed baseline questionnaires between 1 May 2005 and 31 December 2006. Patients were a mean age of 60 years (range, 22–82 years), most were married and most were diagnosed with advanced-stage (FIGO stage III/IV) disease (70%); they had been diagnosed an average of 19 months (range, 3–56 months) previously (Box 1). Twenty-one per cent were currently undergoing treatment. Responders and non-responders were similar in terms of age and marital status. Responders were more likely to have a university education (16%) than non-responders (10%), and, not surprisingly, responders were less likely to have advanced-stage disease at diagnosis (70%) than non-responders (77%). Of the nominated caregivers, 373 (88%) completed baseline questionnaires. Caregivers were a mean age of 58 years (range, 20–86 years) and 72% were male spouses (Box 1).
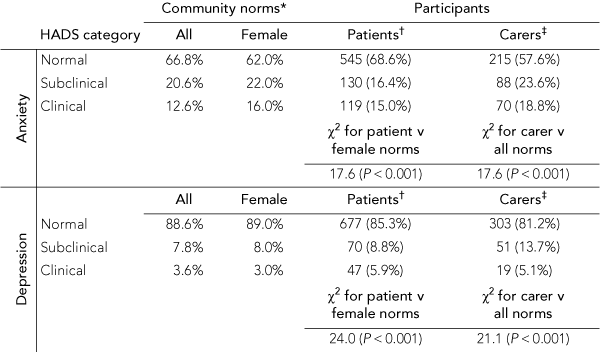
The proportions of patients and caregivers categorised as having normal, subclinical or clinical levels of anxiety and depression were all significantly different to community norms. Box 2 shows that, while clinical levels of anxiety in patients were comparable to female norms (patients, 15.0% v norms, 16.0%), patients reported less subclinical anxiety than female norms (patients, 16.4% v norms, 22.0%; χ2[3] = 17.6; P < 0.001). Conversely, while comparable on subclinical depression (patients, 8.8% v norms, 8.0%), the rate of clinical depression in patients was double that for community norms (patients, 5.9% v norms, 3.0%; χ2[3] = 24.0; P < 0.001).
The results of multivariate analyses of demographic, medical and psychosocial predictors of subclinical or clinical anxiety and subclinical or clinical depression in patients and caregivers are shown in Box 3. For patients, lower optimism, higher symptom burden and current mental health treatment were significant predictors of both anxiety and depression, while lower social support was a significant predictor of patient anxiety only. Demographic variables, including place of residence and medical variables (including stage, treatment and time since diagnosis) were not associated with anxiety or depression. For caregivers, lower social supportand lower optimism were independent predictors of both anxiety and depression. Younger age was an additional predictor of caregiver anxiety (not depression) and current mental health treatment of the patient was an additional predictor of caregiver depression (not anxiety), while children caring for a patient reported significantly lower depression than a partner caring for a patient.
Levels of anxiety and depression reported in our large and representative cohort of women with ovarian cancer are lower than previously reported in this patient group. Earlier studies reported rates of clinical anxiety of 22%–29% and rates of clinical depression of 17%–21%, compared with our findings of 15% for clinical anxiety and 5.9% for clinical depression (although the latter figure is double the rate for community norms23).
Our finding of lower rates of anxiety and depression compared with previous studies may be because we had a more representative, population-based sample in our study. However, given our 66% response rate, it is possible that non-responders were more anxious or depressed. Alternatively, improved recognition of mental health problems in the general community may be in part responsible for the lower rates of anxiety and depression among our participants. In this sample, 49% of patients with clinical levels of anxiety or depression acknowledged a mental health problem, and 69% of these patients (33% of patients with clinical anxiety or depression) reported receiving specialist mental health treatment in addition to other support services. This is a higher uptake of mental and supportive services than the 25% suggested by earlier studies,15 and is an encouraging trend.
Somewhat surprisingly, among demographic, disease and treatment variables, only symptom burden was associated with anxiety and depression in our sample. This contrasts with our expectation that patients receiving active treatment and those with advanced stage disease would report higher levels of distress.24 One explanation may be the nature of ovarian cancer, with most women diagnosed with advanced-stage disease and a high rate of disease progression, potentially making symptom burden the most distinguishing clinical factor among women.
While current mental health treatment is a marker for current anxiety or depression, it may also reflect the success of ongoing mental health treatment in reducing symptoms of anxiety or depression, and it is therefore important to consider current mental health treatment as a potential predictor. Unsurprisingly, patients receiving mental health treatment were more likely to have elevated anxiety and depression. Among other predictors, lower social support predicted higher anxiety, and lower optimism was associated with both anxiety and depression; this is consistent with previous findings.25 These findings suggest potential avenues for intervention targeting the increased use of social support groups and coping styles that promote optimism.
With respect to place of residence, our data are somewhat encouraging. Contrary to expectation,26 we found no differences between patients living in major cities and those in regional/remote locations in terms of the prevalence of anxiety and depression, or the proportion of patients using specialist mental health treatment or support services, although there were some differences in the profile of the types of support services used. Given the growing awareness of the needs of patients in regional/remote areas,27 and the increase in use of telecounselling, support groups, and online services, the divide between city and regional/remote patients may no longer be as great as it was in the past.
The situation of caregivers is less encouraging. Over 42% of caregivers reported elevated anxiety, with 19% scoring within the clinical range. Almost 19% of caregivers also reported elevated levels of depression, with 5% scoring within the clinical range. These levels of caregiver anxiety and depression are significantly higher than community norms. Furthermore, caregivers in our study were significantly more anxious than patients, while caregiver and patient depression levels were similar, consistent in part with previous findings.27 We acknowledge the limitation of caregiver participation in this study (less than half that of patients). It is difficult to estimate how representative our findings are, given that our access to caregivers was dependent on the patients. One of the difficulties in assessing caregivers, and in helping caregivers access appropriate services, is that although they are closely involved in the cancer journey, they are not a primary focus of health services. It is also a limitation of our study that we did not ask caregivers about mental health difficulties and treatment, or the use of other support services.
Predictors of caregiver anxiety and depression included lower social support and lower optimism, consistent with both previous studies6 and the findings for patients. This reinforces the need for support groups to welcome or provide exclusive support to caregivers as well as patients. Because of our patient population, our care-givers were primarily older male spouses, which meant that associations with age and sex could not be explored. Of note, patient distress was not a predictor of caregiver anxiety or depression, and this is also consistent with previous studies.6 Thus, we cannot assume that the caregivers of patients who are distressed will also be the ones in most need, so caregivers need to be assessed independently of the patient.
1 Demographic, disease and treatment, psychosocial, and support variables for 798 patients* and 373 carers*
2 Participants categorised as having normal, subclinical and clinical anxiety and depression on the Hospital Anxiety and Depression Scale (HADS), and comparison with community norms
 |
|||||
- Melanie A Price1,2
- Phyllis N Butow1
- Daniel S J Costa2
- Madeleine T King2
- Lynley J Aldridge2
- Joanna E Fardell1
- Anna DeFazio3,4
- Penelope M Webb5
- , the Australian Ovarian Cancer Study Group and the Australian Ovarian Cancer Study Group Quality of Life Study Investigators
- 1 Centre for Medical Psychology and Evidence-based Decision-making, School of Psychology, University of Sydney, Sydney, NSW.
- 2 Psycho-Oncology Co-operative Research Group, University of Sydney, Sydney, NSW.
- 3 Department of Obstetrics and Gynaecology, University of Sydney, Sydney, NSW.
- 4 Gynaecological Oncology Research Group, Westmead Institute for Cancer Research, Westmead Hospital, Sydney, NSW.
- 5 Genetics and Population Health Division, Queensland Institute of Medical Research, Brisbane, QLD.
This study was funded by the Cancer Councils of New South Wales and Queensland (grant number RG 36/05 New South Wales). Financial support for the parent study was provided by the US Army Medical Research and Materiel Command (grant number DAMD17-01-1-0729), the National Health and Medical Research Council (NHMRC; grant numbers 400413, 400281) and the Cancer Councils of NSW, Queensland, South Australia, Tasmania, Victoria and Western Australia. Additional recruitment was conducted under the Australian Cancer Study (Ovarian Cancer), funded by the NHMRC (199600). Phyllis Butow is supported by an NHMRC Principal Research Fellowship (211199, 457093). Penelope Webb is supported by a Senior Research Fellowship from the NHMRC (496625). Full membership of the Australian Ovarian Cancer Study Group is listed at http://www.aocstudy.org/. We gratefully acknowledge the contribution of all our clinical and scientific collaborators and the cooperation of the following institutions: New South Wales — John Hunter Hospital, North Shore Private Hospital, Royal Hospital for Women, Royal North Shore Hospital, Royal Prince Alfred Hospital, Westmead Hospital, NSW Cancer Registry; Queensland — Mater Misericordiae Hospital, Royal Brisbane and Women’s Hospital, Townsville Hospital, Wesley Hospital, Queensland Cancer Registry; South Australia — Flinders Medical Centre, Queen Elizabeth Hospital, Royal Adelaide Hospital, Burnside War Memorial Hospital, South Australian Cancer Registry; Tasmania — Royal Hobart Hospital; Victoria — Freemasons Hospital, Mercy Hospital for Women, Royal Women’s Hospital, Victorian Cancer Registry; Western Australia — King Edward Memorial Hospital, St John of God Hospital (Subiaco), Sir Charles Gairdner Hospital, Western Australia Research Tissue Network (WARTN), Western Australian Cancer Registry. We also acknowledge the contribution of the study nurses and research assistants and would like to thank all of the women and caregivers who participated in the study.
None identified.
- 1. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia 2000. Cancer series no. 23. Canberra: AIHW, 2003. (AIHW Cat. no. CAN 18.) http://www.aihw.gov.au/publications/index.cfm/title/9469 (accessed Jun 2010).
- 2. National Breast Cancer Centre and National Cancer Control Initiative. Clinical practice guidelines for the psychosocial care of adults with cancer. Sydney: National Breast Cancer Centre, 2003.
- 3. Holland JC, Andersen B, Breitbart WS, et al. Distress management: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2007; 5: 66-98.
- 4. Breen SJ, Baravelli CM, Schofield PE, et al. Is symptom burden a predictor of anxiety and depression in patients with cancer about to commence chemotherapy? Med J Aust 2009; 190 (7 Suppl): S99-S104. <MJA full text>
- 5. Arden-Close E, Gidron Y, Moss-Morris R. Psychological distress and its correlates in ovarian cancer: a systematic review. Psychooncology 2008; 17: 1061-1072.
- 6. Pitceathly C, Maguire P. The psychological impact of cancer on patients’ partners and other key relatives: a review. Eur J Cancer 2003; 39: 1517-1524.
- 7. Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psychooncology 2004; 13: 562-576.
- 8. Carlson LE, Bultz BD. Benefits of psychosocial oncology care: improved quality of life and medical cost offset. Health Qual Life Outcomes 2003; 1: 8.
- 9. Matthews EE, Cook PF. Relationships among optimism, well-being, self-transcendence, coping, and social support in women during treatment for breast cancer. Psychooncology 2009; 18: 716-726.
- 10. Brothers BM, Andersen BL. Hopelessness as a predictor of depressive symptoms for breast cancer patients coping with recurrence. Psychooncology 2009; 18: 267-275.
- 11. Hoffman KE, McCarthy EP, Recklitis CJ, et al. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med 2009; 169: 1274-1281.
- 12. Steginga SK, Campbell A, Ferguson M, et al. Socio-demographic, psychosocial and attitudinal predictors of help seeking after cancer diagnosis. Psychooncology 2008; 17: 997-1005.
- 13. Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: analyses of the 1992 national health interview survey. J Natl Cancer Inst 1999; 91: 1480-1486.
- 14. Owen JE, Goldstein MS, Lee JH, et al. Use of health-related and cancer-specific support groups among adult cancer survivors. Cancer 2007; 109: 2580-2589.
- 15. Pascoe S, Edelman S, Kidman A. Prevalence of psychological distress and use of support services by cancer patients at Sydney hospitals. Aust N Z J Psychiatry 2000; 34: 785-791.
- 16. Glasdam S, Jensen AB, Madsen EL, et al. Anxiety and depression in cancer patients’ spouses. Psychooncology 1996; 5: 23-29.
- 17. Begbie S, Underhill C. Cancer services to be proud of in rural Australia: lessons learnt from the Clinical Oncological Society of Australia Cancer Service audit. Cancer Forum 2007; 31: 90-93.
- 18. Jordan SJ, Green AC, Whiteman DC, et al. Serous ovarian, fallopian tube and primary peritoneal cancers: a comparative epidemiological analysis. Int J Cancer 2008; 122: 1598-1603.
- 19. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361-370.
- 20. Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy — ovarian. J Clin Oncol 2001; 19: 1809-1817.
- 21. Broadhead WE, Gehbach SH, de Gruy FV, et al. The Duke-UNC Functional Social Support Questionnaire. Med Care 1988; 26: 709-723.
- 22. Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self mastery, and self esteem): a reevaluation of the life orientation test. J Pers Soc Psychol 1994; 67: 1063-1078.
- 23. Crawford JR, Henry JD, Crombie C, et al. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 2001; 40: 429–434.
- 24. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology 2001; 10: 19-28.
- 25. Mirabeau-Beale KL, Kornblith AB, Penson RT, et al. Comparison of the quality of life of early and advanced stage ovarian cancer survivors. Gynecol Oncol 2009; 114: 353-359.
- 26. Bettencourt BA, Schlegel RJ, Talley AE, et al. The breast cancer experience of rural women: a literature review. Psychooncology 2007; 16: 875-887.
- 27. Clinical Oncological Society of Australia. Publications. Mapping rural and regional oncology services in Australia. March 2006. http://www.cosa.org.au//PublicationsPositionStatements/Publications.htm (accessed April 2010).





Abstract
Objectives: To assess the prevalence and predictors of depression and anxiety in women with ovarian cancer and their caregivers, to compare levels of depression and anxiety with community norms, and to explore the relationship between patients and their nominated caregivers.
Design, setting and participants: Prospective cohort study of 798 women with invasive ovarian cancer recruited between 1 January 2002 and 30 June 2006 through the nationwide Australian Ovarian Cancer Study, and 373 of their caregivers.
Main outcome measures: Depression and anxiety as assessed with the Hospital Anxiety and Depression Scale, and the role of demographic variables, disease and treatment variables, psychosocial variables, and use of mental health and support services as potential predictors.
Results: Rates of anxiety and depression among patients were significantly lower than in previous reports, although clinical depression rates (5.9%) were significantly higher than community norms (3.0%; χ2 = 24.0; P < 0.001). Caregivers also reported higher levels of depression (χ2 = 21.1; P < 0.001) and anxiety (χ2 = 17.6; P < 0.001) compared with norms. There was no difference within patient–caregiver pairs for depression (P = 0.1), while caregivers reported significantly higher anxiety than patients (P < 0.01). In patients, higher symptom burden, lower optimism and current specialist mental health treatment all significantly predicted both depression and anxiety, while lower social support was a significant predictor of patient anxiety only. In caregivers, lower social support and lower optimism were significant predictors of depression and anxiety. Patients being treated for mental health was also a predictor of their caregiver’s depression.
Conclusions: While depression is significantly more common in women with ovarian cancer than in the general population, it is caregivers of such patients who report much higher levels of both subclinical and clinical depression and anxiety.