In Australia, oesophageal cancer represents 1.2% of all cancers and is responsible for 2.1% of cancer deaths. Recently, there has been a striking increase in oesophageal adenocarcinoma (OAC) incidence, estimated at 4.2% per year in New South Wales,1 whereas the incidence of oesophageal squamous cell carcinoma (OSCC) has declined. Almost all of the increase in OAC incidence has occurred in males, contributing to a male–female ratio approaching 8 to 1.1 Gastro-oesophageal junction adenocarcinoma (GOJAC) has also increased in incidence. The incidence patterns for OAC and GOJAC contrast with those for OSCC. This parallels trends observed in other Western countries2,3 and is not due to changes in diagnostic criteria.4 The principal causes of the increase in OAC (and probably GOJAC) are thought to be increased prevalence of gastro-oesophageal acid reflux and obesity in Western populations.5-11 Changing patterns of obesity appear to be driving the rising incidence of OAC, with particular attention focusing on “male pattern” central adiposity, which is postulated to increase the production of mitogenic, obesity-related hormones.12,13 Falling rates of Helicobacter pylori infection may also play a role, as chronic infection causes hypochlorhydria and thus protects against reflux-mediated carcinogenesis.14
The prognosis for patients diagnosed with these cancers is poor; 1-year survival for patients with OAC in a NSW study was 49% for localised cancer, 43% for cancers with regional spread and 12% for disseminated cancers.1 Yet despite the rapid increases in incidence and the poor survival from oesophageal cancers, relatively little is known about the patterns of care for patients with these diseases. Here we report the findings of an investigation into the presentation and clinical management of a cohort of patients with carcinomas of the oesophagus or gastro-oesophageal junction.
Our study was based on a cohort of patients previously enrolled in the Australian Cancer Study (ACS), a population-based, case–control study undertaken to investigate risk factors for oesophageal cancer.6 For our study (the ACS Clinical Follow-up Study), we collected clinical information and outcome data on ACS patients. We collected data on the presenting symptoms of patients, investigations and treatment pathways.
For the ACS, all patients aged 18–79 years with a histologically confirmed primary invasive cancer of the oesophagus or gastro-oesophageal junction diagnosed between 1 July 2002 (1 July 2001 in Queensland) and 30 June 2005 in mainland Australia were identified. Full details of recruitment of patients into the ACS have been described elsewhere.6 Briefly, patients were ascertained principally via systematic review of admissions and clinic registers at major treatment centres throughout Australia; additional cases were identified by cancer registries (cancer notification is mandatory in all states). Histological details were abstracted from pathology reports. Anatomical sites of adenocarcinoma tumours were categorised according to the World Health Organization classification into “oesophageal” and “oesophago-gastric junction” tumours.15 For analysis, we compared patients with OAC, GOJAC and OSCC.
Thus, patients self-completed a questionnaire on recruitment into the ACS (2002–2005), followed shortly after by a standardised interview to elicit details of symptom history, presentation, and pathway to diagnosis. Case–control analyses to identify risk factors for oesophageal cancer have been reported separately.5-7
We assigned each participant an index of remoteness and accessibility to services based on their residential postcode using the 2006 Accessibility/Remoteness Index of Australia codes from the Australian Government Department of Health and Ageing (http://www9.health.gov.au/aria/ariainpt.cfm).
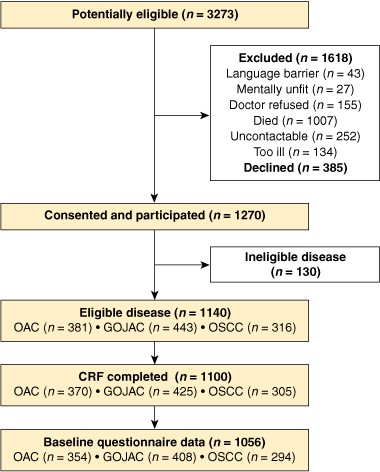
We identified 3273 potentially eligible patients with oesophageal cancer, of whom 1618 were excluded for various reasons and 385 declined to participate (Box 1). Of the remaining 1270, 1140 patients had histologically confirmed oesophageal cancer and gave consent for access to their medical records. Completed case report forms were available for 1100 patients (370 OAC, 425 GOJAC and 305 OSCC) and linked questionnaire data were available for 1056 patients.
Patient demographics are shown in Box 2. Notably, age distributions were similar for the three patient groups, whereas sex distributions were markedly different for OSCC patients compared with OAC and GOJAC patients. Eighty-seven per cent of patients resided in cities and towns, 11% lived in moderately accessible regional locations, and 2% were from remote or very remote locations.
Interview data describing medical presentation and symptom history were available for 831 patients. The primary symptom for which the patient sought medical attention, the prevalence of all symptoms volunteered by the patient, and the prevalence of symptoms as elicited and recorded by the doctor are shown in Box 3. Dysphagia, the most frequent primary symptom, was self-reported by 41%–48% of patients. Gastro-oesophageal acid reflux was self-reported by 7%–9% of patients as the primary reason for presentation but elicited by a doctor in 46% of OAC and 44% of GOJAC patients. As recorded by the doctor, OSCC patients had a higher prevalence of odynophagia than OAC or GOJAC patients, but less reflux. Odynophagia, epigastric pain, chest pain and weight loss were all uncommon reasons for presentation, but were commonly found to be present on direct questioning. Eight per cent and 3% of OAC and GOJAC patients, respectively, were diagnosed through Barrett’s oesophagus surveillance programs, and 2%–4% of OAC, GOJAC and OSCC diagnoses were incidental findings from routine health checks.
All patients had undergone upper gastrointestinal endoscopy as an eligibility criterion for our study (Box 4). A computed tomography (CT) scan was performed in 93%–95% of patients, a fluorodeoxyglucose positron emission tomography (FDG-PET) scan in 42%–51% of patients, and endoscopic ultrasound (EUS) in 20%–21% of patients. Laparoscopy was more commonly performed in the GOJAC group than the OAC and OSCC groups.
An AJCC stage was reported in 7% of patient records (range, 5%–10%) (Box 5); converting tumour–node–metastasis (TNM) codes into AJCC tumour stages increased the overall proportion of patients with stage data to 25% (range, 23%–27%). Imputation using the FDG-PET scan result for M status and EUS for T and N status increased the overall proportion of patients with stage data to 49% (range, 47%–50%). However, for about half of the patients, there were insufficient data to estimate pretreatment cancer stage.
Curative treatments were attempted for 60% (222/370) of OAC, 88% (372/425) of GOJAC, and 65% (197/305) of OSCC patients. Overall, 72% of patients were treated with curative intent, of whom the majority (61%) had surgical resection (Box 6). Of patients offered curative therapy, those with OAC or GOJAC were more likely to have surgery than those with OSCC. Among surgical patients, preoperative (neoadjuvant) therapy was performed on similar proportions of patients with OAC, GOJAC and OSCC. Preoperative chemoradiotherapy (CRT) was used more commonly than preoperative chemotherapy alone. Postoperative therapy was performed most frequently for patients with GOJAC followed by patients with OSCC and those with OAC.
The most common presenting symptom was dysphagia, which on direct questioning was found to be present in over 70% of patients, a proportion similar to that found in other studies.16,17 Dysphagia occurs when the oesophageal circumference has been reduced by two-thirds,18 which is sufficient to compromise the lumen. The United Kingdom guidelines for managing oesophageal cancer outline a number of “alarm symptoms”, of which dysphagia is the first, and for which referral for endoscopy is recommended within 2 weeks of presentation.19 Our data suggest most patients do not recognise the importance of dysphagia as an alarm symptom. Only 7%–9% of patients reported reflux as their primary symptom, a similar proportion to that reported in a previous study.16 The underlying precipitant for these patients may have been a change, likely worsening, of previous reflux symptoms or the development of new symptoms.
A key finding was the infrequent recording of AJCC pretreatment tumour stage (7%), and although stage could be imputed for a further 42%, for about half of the patients it was impossible to determine the extent of their cancer. Of the staging investigations employed, CT scans were the most common, being used in more than 90% of patients in our study. The major benefit of CT is the ability to rapidly identify patients with distant metastases. Staging information is improved by also performing an EUS to assess local infiltration and local nodal status. Relatively few Australian centres were performing EUS at the time of our study, hence the low usage we observed. Compared with conventional staging modalities, FDG-PET scanning has been shown to detect distant metastases in 4%–28% of oesophageal cancer patients and to change management in 3%–40% of patients.20 Although fewer centres were performing FDG-PET than EUS in Australia at the time of our study, FDG-PET scans were reported for 42%–51% of the patients. Thus, despite limited availability, both FDG-PET scanning and EUS were performed on sizeable numbers of patients, suggesting rapid uptake for these modalities.
Putting aside the extent of incomplete reporting, our study differs from others in having relatively fewer patients with stage IV cancers. For example, a United States study reported stage IV disease in 48% of OAC patients and 52% of GOJAC patients21 — considerably higher than the proportions we observed. Reporting of cancer stage is not mandatory in Australia, hence there are no reliable population-based data for comparison. Instead, estimates of the distribution of cancer stage must be derived from chart reviews. As the use of chart reviews requires patient consent, and because consent is less likely among patients with late-stage disease, it is likely that all studies based on chart review will underestimate the incidence of late-stage disease.
We identified apparent differences in the surgical management of OAC and GOJAC. Specifically, our data suggest that patients with GOJAC are more likely to undergo surgery alone than OAC or OSCC patients. A similar finding was reported in an Irish study.22 Information bias may partly explain these differences, as the location of a tumour can be identified more precisely from surgical resection specimens than from endoscopy. Thus, patients who have undergone surgery are more likely to have the anatomical location of their tumour classed as gastro-oesophageal junction than patients who have not received surgery.
The proportion of surgical patients undergoing preoperative (neoadjuvant) therapy in our study was lower than the proportion in the US study21 but higher than that found in the Irish study.22 At the time that the patients in our study were being treated, there had been one international report of benefit from neoadjuvant CRT in patients with OAC,23 and one account of benefit from preoperative chemotherapy in both OAC and OSCC patients.24 An Australian phase II study assessing the role of neoadjuvant CRT and CRT alone for cure and palliation of OAC and OSCC had also been reported before our assessment.25 It is likely that these publications, along with an active Australian trial of preoperative CRT,26 raised awareness among Australian clinicians who treat such patients. This could explain the high prevalence of neoadjuvant therapy for OAC and OSCC, and may also explain why 10% of patients in our study were also enrolled in trials. While there is no evidence from trials that routine postoperative therapy improves survival, we observed reasonably high proportions of patients undergoing postoperative therapy. The reasons for this were not clear from the records.
We found that use of definitive CRT in our study was markedly higher for patients with OSCC (37%) than for patients with OAC (13%) or GOJAC (5%). In comparison, the Irish study observed proportions of patients undergoing definitive CRT as 12% for OAC, 6% for GOJAC and 12% for OSCC.22 A possible explanation for the difference may be a widespread perception among clinicians that adenocarcinomas are less sensitive to radiation than squamous cell carcinomas. There are limited data to determine the validity of this perception; however, there are more publications reporting benefits for CRT as an alternative to resection for OSCC27,28 than as a treatment for OAC.
3 ACS Clinical Follow-up Study: reasons for presentation self-reported by patient* and recorded by doctor in clinical files
Primary reason self-reported by patient |
All reasons self-reported by patient |
All reasons recorded by doctor† |
|||||||||||||
5 ACS Clinical Follow-up Study: pretreatment staging
Imputed from doctor’s record of TNM codes and clinical test results |
|||||||||||||||
Received 1 March 2010, accepted 3 August 2010
- Bernard M Smithers1
- Paul P Fahey2
- Tracie Corish2
- David C Gotley1
- Gregory L Falk3
- Garett S Smith4
- George K Kiroff5
- Andrew D Clouston6
- David I Watson7
- David C Whiteman2
- 1 Upper GI and Soft Tissue Unit, Division of Surgery, Princess Alexandra Hospital, Brisbane, QLD.
- 2 Queensland Institute of Medical Research, Brisbane, QLD.
- 3 Concord Repatriation General Hospital, Sydney, NSW.
- 4 University of Sydney Northern Clinical School, Royal North Shore Hospital, Sydney, NSW.
- 5 Geelong Hospital, Geelong, VIC.
- 6 Envoi Specialist Pathologists, Brisbane, QLD.
- 7 Department of Surgery, Flinders University, Adelaide, SA.
Our study was supported by the Cancer Council Queensland and the National Health and Medical Research Council. David Whiteman is supported by a Future Fellowship from the Australian Research Council. The funding bodies played no role in the design or conduct of the study; the collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript. We thank Shahram Sadeghi for assistance with pathology abstractions, and Nirmala Pandeya for data cleaning and programming. The study investigators were David Whiteman, Adele Green, David Gotley, B Mark Smithers, David Watson, Gregory Falk, Garett Smith, George Kiroff, Steven Archer, Nicholas Hayward and Andrew Clouston; the project manager was Tracie Corish; the database managers were Karen Harrap and Troy Sadkowski; and the research nurses were Janine Thomas, Ellen Minehan, Deborah Roffe, Sue O’Keefe, Suzanne Lipshut, Gabby Connor, Hayley Berry, Linda Terry, Michael Connard, Leanne Bowes, MaryRose Malt and Jo White. The clinical contributors were: Australian Capital Territory — Charles Mosse and Noel Tait; New South Wales — Chris Bambach, Andrew Biankan, Roy Brancatisano, Max Coleman, Michael Cox, Stephen Deane, James Gallagher, Mike Hollands, Tom Hugh, David Hunt, John Jorgensen, Christopher Martin, Mark Richardson, Ross Smith and David Storey; Queensland — John Avramovic, John Croese, Justin D’Arcy, Stephen Fairley, John Hansen, John Masson, Ian Martin, Les Nathanson, Barry O’Loughlin, Leigh Rutherford, Richard Turner and Morgan Windsor; South Australia — Justin Bessell, Peter Devitt and Glyn Jamieson; Victoria — Stephen Blamey, Alex Boussioutas, Richard Cade, Gary Crosthwaite, Ian Faragher, John Gribbin, Geoff Hebbard, Bruce Mann, Bob Millar, Paul O’Brien, Robert Thomas and Simon Wood; Western Australia — Kingsley Faulkner and Jeff Hamdorf.
None identified.
- 1. Stavrou EP, McElroy HJ, Baker DF, et al. Adenocarcinoma of the oesophagus: incidence and survival rates in New South Wales, 1972–2005. Med J Aust 2009; 191: 310-314. <MJA full text>
- 2. Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83: 2049-2053.
- 3. Botterweck AA, Schouten LJ, Volovics A, et al. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol 2000; 29: 645-654.
- 4. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97: 142-146.
- 5. Pandeya N, Webb PM, Sadeghi S, et al. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut 2010; 59: 31-38.
- 6. Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2008; 57: 173-180.
- 7. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008; 168: 105-114.
- 8. Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998; 90: 150-155.
- 9. Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999; 130: 883-890.
- 10. Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr 2002; 132 (11 Suppl): 3467S-3470S.
- 11. Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995; 4: 85-92.
- 12. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 2006; 207: 12-22.
- 13. Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut 2008; 57: 448-454.
- 14. Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev Res (Phila) 2008; 1: 308-311.
- 15. Spechler SJ, Dixon MF, Genta R, et al. Adenocarcinoma of the oesophago-gastric junction. In: Hamilton SR, Aaltonen LA, editors. Pathology and genetics of tumours of the digestive system. World Health Organization classification of tumours. Volume 2. Lyon: IARC Press, 2000.
- 16. Gibbs JF, Rajput A, Chadha KS, et al. The changing profile of esophageal cancer presentation and its implication for diagnosis. J Natl Med Assoc 2007; 99: 620-626.
- 17. Bytzer P, Christensen PB, Damkier P, et al. Adenocarcinoma of the esophagus and Barrett’s esophagus: a population-based study. Am J Gastroenterol 1999; 94: 86-91.
- 18. DeMeester TR, Barlow AP. Surgery and current management for cancer of the esophagus and cardia: part I. Curr Probl Surg 1988; 25: 475-531.
- 19. Allum WH, Griffin SM, Watson A, Colin-Jones D. Guidelines for the management of oesophageal and gastric cancer. Gut 2002; 50 Suppl 5: v1-v23.
- 20. Salavati A, Basu S, Heidari P, Alavi A. Impact of fluorodeoxyglucose PET on the management of esophageal cancer. Nucl Med Commun 2009; 30: 95-116.
- 21. Cronin-Fenton DP, Mooney MM, Clegg LX, Harlan LC. Treatment and survival in a population-based sample of patients diagnosed with gastroesophageal adenocarcinoma. World J Gastroenterol 2008; 14: 3165-3173.
- 22. Cronin-Fenton DP, Sharp L, Carsin AE, Comber H. Patterns of care and effects on mortality for cancers of the oesophagus and gastric cardia: a population-based study. Eur J Cancer 2007; 43: 565-575.
- 23. Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462-467.
- 24. Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; 359: 1727-1733.
- 25. Burmeister BH, Denham JW, O’Brien M, et al. Combined modality therapy for esophageal carcinoma: preliminary results from a large Australasian multicenter study. Int J Radiat Oncol Biol Phys 1995; 32: 997-1006.
- 26. Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005; 6: 659-668.
- 27. Chiu PW, Chan AC, Leung SF, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). J Gastrointest Surg 2005; 9: 794-802.
- 28. Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005; 23: 2310-2317.





Abstract
Objective: To document presenting symptoms, investigations and management for Australian patients with oesophageal adenocarcinoma (OAC), gastro-oesophageal junction adenocarcinoma (GOJAC) and oesophageal squamous cell carcinoma (OSCC).
Design, setting and participants: Cross-sectional study of a population-based sample of 1100 Australian patients aged 18–79 years with histologically confirmed oesophageal cancer diagnosed in 2002–2005, using data from cancer registries and treatment centres, supplemented with clinical information collected through medical record review in 2006–2007 and mortality information collected in 2008.
Main outcome measures: Prevalence of primary symptoms, and staging investigations and treatment modalities used.
Results: The primary presenting symptom was dysphagia, which was self-reported by 41%, 39% and 48% of patients with OAC, GOJAC and OSCC, respectively. Less common symptoms were reflux, chest pain, bleeding and weight loss. All patients underwent endoscopy, most had a staging computed tomography scan (OAC 93%, GOJAC 95% and OSCC 93%), and about half had positron emission tomography scans (OAC 51%, GOJAC 44% and OSCC 42%). Pretreatment tumour stage was reported in 25% of records, and could be derived from results of investigations in a further 23%, but the remaining half lacked sufficient information to ascribe a pretreatment stage. Curative treatments were attempted for 60% of OAC, 88% of GOJAC and 65% of OSCC patients. Surgery was performed on 52% of OAC, 83% of GOJAC and 41% of OSCC patients. About two-thirds of surgical patients received additional therapies.
Conclusions: With anticipated increases in oesophageal cancer incidence, the resources required to diagnose and manage patients with oesphageal cancer are also likely to rise. Our data provide a baseline from which to plan for the future care of patients with cancers of the oesophagus.