Aortic stenosis is the most common acquired valvular heart disease in Western countries, and its prevalence increases with advancing age.1 Over the past century, the life expectancy of adults in Western countries has increased significantly, resulting in a greater proportion of patients over the age of 80 years.2 Accompanying this social change has been an increase in the number of octogenarian patients with an indication for aortic valve replacement (AVR) being referred for cardiac surgery.2
Surgical AVR is the definitive treatment for aortic valve disease. It is a proven treatment, with extremely good long-term follow-up data, that relieves symptoms and improves prognosis in successfully treated patients.3 However, older patients, with their increasing prevalence of comorbidities, are often overlooked for AVR because of a perceived increased risk.4 Analysis of data on over 5000 patients from the Euro Heart Survey on valvular heart disease found that a third of elderly patients with severe, symptomatic aortic stenosis were denied valve surgery.5 There is a lack of suitable treatment alternatives for such patients.
Until the 1980s, surgical AVR was the only effective treatment for severe aortic stenosis. The first report on adult BAV, in three patients, was published in 1986 with promising results,6 and it was initially thought that valvuloplasty would be a viable alternative to, if not a replacement for, surgical AVR. Acutely, BAV reduced aortic valve gradient, increased calculated aortic valve area and improved left ventricular ejection fractions.6 It was also associated with improved functional outcomes. Based on these original findings, many BAV procedures were performed worldwide during the mid–late 1980s and early 1990s.
However, the results from several large registries in the 1980s challenged the long-term efficacy of the treatment.7-9 There was compelling evidence that BAV did not alter the natural history of symptomatic stenosis and had poor long-term outcomes, with valve restenosis occurring in a majority of patients by 6 months.7 Limited evidence suggested that repeat BAV may offer improved survival at 3-year follow-up, but this was based on small numbers of highly selected patients and the results may therefore not be generalisable.10 A more recent review of 80 consecutive patients with severe, symptomatic aortic stenosis, who were declined surgery due to high-risk features, demonstrated good initial procedural success with BAV, with no procedure-related deaths. However, the mortality at 3 years was 71%, implying that, despite improvements in technique, valvuloplasty was not the definitive treatment for symptomatic aortic stenosis.11 Indeed, guidelines recommend that BAV should be reserved for palliative measures or as a bridge to surgery in haemodynamically unstable patients.3
The development of a percutaneous bioprosthetic heart valve provided a new therapeutic option for patients with severe aortic stenosis. Percutaneous valve replacement was successfully demonstrated in pig models in 1992,12 but it was not until 2002 that the first percutaneous aortic catheter-mounted bioprosthetic valve was deployed in a human, in a 57-year-old man with critical aortic stenosis and cardiogenic shock.13 Although there was immediate haemodynamic improvement, the patient died 17 weeks later of unrelated causes; however, valve function was preserved.13 Following this initial pioneering effort led by French cardiologist Alain Cribier, there has been a dramatic growth in the development of percutaneous valve technologies.
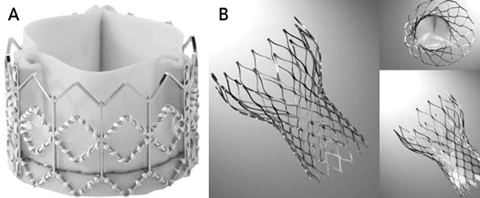
There are two main percutaneous valves available — the Edwards SAPIEN valve, which superseded the Cribier-Edwards valve (Edwards Lifesciences, Irvine, Calif, USA) (Box 1, A), and the CoreValve ReValving System (CoreValve, Irvine, Calif, USA) (Box 1, B). The technical specifications for each valve and the main differences between them are summarised in Box 2. The most significant differences are that the Edwards SAPIEN valve is balloon-expandable, requiring rapid ventricular pacing for safe deployment, and is placed in the subcoronary position, whereas the CoreValve is self-expanding and placed in a supra-annular position, without the need for rapid pacing. The delivery sheaths for the Edwards SAPIEN valve have generally been much larger than those for the CoreValve, meaning that access site problems may be more frequent with the Edwards system.15
There are several ways to deploy these valves — antegrade, retrograde and, more recently, transapically — and each method has its individual merits (Box 3). The antegrade approach was initially favoured, but issues with procedural complexity and mitral valve disruption led to widespread adoption of the retrograde approach.
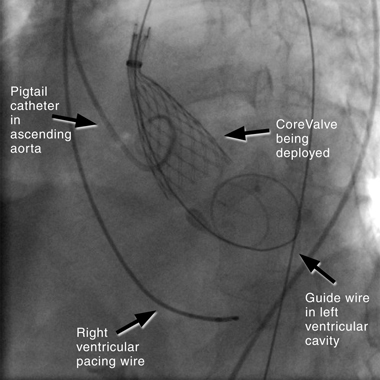
The retrograde approach has undergone rapid modification in recent years, due to the development of specialised closure devices that have allowed for a percutaneous procedure without the need for formal femoral surgical repair.16 It is now by far the most common approach to PAVR, because it is a simpler technique and has procedural similarities to coronary angiography. The technique (illustrated in Box 4) involves inserting a large sheath (18–22F) into the femoral artery, with a pre-closure device. If the subclavian route is used, a surgical cutdown is required, with direct suture closure of the vessel at the completion of the procedure. A right ventricular pacing wire is placed for rapid pacing (220 beats/min) that is performed during BAV and for temporary cardiac pacing if bradycardia occurs. The aortic valve is crossed using a guide wire, and the device is positioned across the valve. The CoreValve is self-deployed, and the Edwards SAPIEN valve is balloon-deployed under rapid ventricular pacing to reduce left ventricular stroke volume and reduce risk of device migration. The large femoral sheath is removed using the pre-closure device.
In highly selected cohorts in high-volume centres, mortality for surgical AVR in octogenarians can be as low as 11% at 30 days,2 with 1-year survival of 74% found for nonagenarians in the United Kingdom.17 However, despite these impressive outcomes, there is still considerable variability in the management of older patients, due to the increase in comorbidities and therefore risk that often accompanies advancing age.4 In Australia, PAVR is presently only offered to patients who are deemed too high-risk to undergo formal surgical AVR, most of whom are elderly. However, age is only one factor involved in risk assessment.
The European System for Cardiac Operative Risk Evaluation (euroSCORE) is well validated and provides a simple, additive risk model in adult cardiac surgery populations.18 However, studies of octogenarians undergoing AVR have demonstrated that the additive and logistic euroSCORE overestimates mortality in this group. A study of 1545 patients with severe aortic stenosis who underwent isolated AVR found that the predicted mortality was substantially overestimated by the additive and logistic euroSCORE, and no patient with a high euroSCORE actually died.19 Why does the euroSCORE perform so badly in this patient group? The euroSCORE was devised with a mixed population undergoing surgery — most had ischaemic heart disease, with only a minority having isolated AVR.19 It is also based on 1995 data, with a lack of high-risk patients in the original cohort. The risk-adjustment mechanism only accounted for the most prevalent risk factors, such as ejection fraction and advancing age, but failed to include high-risk features such as chronic liver disease or the presence of a “porcelain aorta”, which will influence outcomes.
The alternative and less widely adopted Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score is another well validated tool, originally developed as a risk model for stratifying isolated coronary artery bypass graft patients.20 The score tends to underestimate mortality in general cardiac surgical patients, but performs better than the euroSCORE in high-risk patients undergoing isolated AVR,21 and therefore may be more suited to assessing risk in patients being considered for PAVR.
Frailty is not accounted for in either the euroSCORE or STS PROM score, yet it needs to be taken into consideration when assessing older patients for PAVR. Frailty is increasingly recognised as a very important factor in the postoperative outcomes of geriatric patients, with one study recently demonstrating that increasing frailty predicted 6-month mortality in a cohort of elderly patients undergoing major elective surgery.22 The traditional risk models of euroSCORE and STS PROM, although useful, are based on a younger population, and a tailored geriatric-specific risk assessment may provide a more accurate and realistic tool to assess risk in these patients.
Careful selection of patients is fundamental to successful outcomes with any new technology. For patients undergoing PAVR, particularly with the CoreValve, there is a complex selection process.23 One group who looked at all patients referred to their institution for PAVR found that 14% of the cohort were not eligible for percutaneous treatment because they were deemed too high-risk or because PAVR was not technically feasible.24 Detailed clinical evaluation, together with echocardiography and vascular assessment of the iliofemoral arterial system and proximal aorta — particularly the origin of the coronary arteries and aortic annulus size — is required, often with a combination of formal and computed tomography angiography.
The main published registry data for the Cribier-Edwards, Edwards SAPIEN and Edwards SAPIEN XT valves are summarised in Box 5. Although earlier published cases were performed using the antegrade approach, an improvement in outcomes can be seen as the devices and techniques have undergone improvements and modifications. The most recent data, published in 2009,27 demonstrate a reduction in complications and improved outcomes from the earliest registry data. This reflects the learning curve of the operators, improved patient selection and continually improving techniques. Box 6 summarises the main registry data for the CoreValve ReValving System, and demonstrates a marked improvement in procedural success rates and outcomes between the cohort reported in 200728 and the largest cohort, reported in 2008.23 This again reflects improvements in patient selection and the growing experience of operators.
The relatively newer transapical technique is limited to the Edwards SAPIEN valve at present, although the CoreValve has recently been used safely in an animal model.14 The first transapical cohort registry data were encouraging, demonstrating a 30-day hospital mortality rate of 13.6% and overall mortality at a mean of 110 days of 22% in high-risk elderly patients.31 Recently published 12-month outcomes for another group of patients reported a 30-day mortality of 23%, with overall survival at 1 year of 85%.32 These were high-risk patients, but valve haemodynamics and function were preserved at follow-up, and there was a marked improvement in symptomatic status in surviving patients. The transapical procedure is ideally suited to patients with severe iliofemoral disease that prohibits the use of a transfemoral technique. A significant proportion of the first transapical cohort required cardiopulmonary bypass,31 but greater operator experience enables the technique to be performed without this. As with the transfemoral technique, increased operator experience and improved patient selection are likely to result in improved outcomes.
Complications (see Box 5 and Box 6) were generally higher in the earlier case series. They can arise from any of the steps involved in valve deployment, from gaining vascular access to the BAV performed before placement of the valve. Vascular injury remains a significant complication of the technique and is not isolated to the larger-profile devices. Conduction system disease, and the need for a permanent pacemaker, may occur more often with the CoreValve than the Edwards SAPIEN valve and is thought to relate to the proximal portion of the device encroaching on the membranous septum and possibly the left bundle branch itself.33 The relatively short-term follow-up data that are currently available limit our knowledge about long-term survival, thus curtailing our ability to determine valve durability and future risk of valve degeneration.
Whether PAVR will challenge surgical AVR as the definitive treatment for aortic stenosis will be determined by the longevity of the prostheses and the results of randomised controlled trials. The Edwards PARTNER (Placement of Aortic Transcatheter Valve) trial is a prospective randomised controlled trial with two separate treatment arms that is currently recruiting participants.34 In the first arm, 350 high-risk patients who are candidates for conventional open-heart surgery will be randomly assigned to receive either surgery or the Edwards SAPIEN valve. The hypothesis to be tested is that PAVR will provide some advantage over formal surgical AVR. The other arm of the trial will randomly assign about 250 patients who are considered too high-risk for conventional surgery to receive either the Edwards SAPIEN valve or medical therapy, on the premise that PAVR will provide some advantage to this high-risk group. The primary end point in both arms of the trial is mortality at 1 year, with secondary end points focusing on valve performance and quality-of-life indicators. We hope that the results of this trial will help to scientifically clarify where the clinical utility of PAVR lies.
PAVR has also been used in patients with bioprosthetic valve failure, in both stentless and stented valves, and it may be a viable treatment option for such patients.35 Indeed, this may be one of the main clinical uses of PAVR, as patients with bioprosthetic valve failure often tend to be older and are too high-risk for redo sternotomy. It may also be useful for patients with patent grafts from previous coronary artery bypass grafting who develop aortic stenosis years later.
2 Technical specifications of the available percutaneous aortic valve prostheses
3 Methods of percutaneous aortic valve deployment
4 Fluoroscopic image in anterior–posterior projection showing retrograde deployment of CoreValve ReValving System
- Jamie J Layland1
- Brendan Bell2
- Dan Mullany3
- Darren L Walters4
- Prince Charles Hospital, Brisbane, QLD.
Darren Walters is an investigator in clinical trials for Medtronic CoreValve and Edwards Lifesciences, and an honorary consultant to Edwards Lifesciences.
- 1. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population based study. Lancet 2006; 368: 1005-1011.
- 2. Kolh P, Kerzmann A, Lahaye L, et al. Cardiac surgery in octogenarians: peri-operative outcome and long-term results. Eur Heart J 2001; 22: 1235-1243.
- 3. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e1-e142.
- 4. Bouma BJ, van der Meulen JH, van den Brink RB, et al. Variability in treatment advice for elderly patients with aortic stenosis: a nationwide survey in the Netherlands. Heart 2001; 85: 196-201.
- 5. Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26: 2714-2720.
- 6. Cribier A, Savin T, Saoudi N, et al. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1: 63-67.
- 7. Feldman T, Glagov S, Carroll JD. Restenosis following successful balloon valvuloplasty: bone formation in aortic valve leaflets. Cathet Cardiovasc Diagn 1993; 29: 1-7.
- 8. Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319: 125-130.
- 9. Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89: 642-650.
- 10. Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95: 43-47.
- 11. Shareghi S, Rasouli L, Shavelle DM, et al. Current results of balloon aortic valvuloplasty in high-risk patients. J Invasive Cardiol 2007; 19: 1-5.
- 12. Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13: 704-708.
- 13. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106: 3006-3008.
- 14. Kappetein AP, Piazza N, Laborde JC, et al. Transapical implantation of a self-expanding aortic valve bioprosthesis — animal feasibility study. Eur J Cardiothorac Surg 2009; 36: 813-817.
- 15. Dworakowski R, MacCarthy P. Transcatheter aortic valve implantation (TAVI) — a novel treatment for critical aortic stenosis. http://www.pinpointmedical.com/uploads/pdfs/1313A.pdf (accessed Mar 2010).
- 16. Kahlert P, Eggebrecht H, Erbel R, Sack S. A modified “preclosure” technique after percutaneous aortic valve replacement. Catheter Cardiovasc Interv 2008; 72: 877-884.
- 17. Edwards MB, Taylor KM. Outcomes in nonagenarians after heart valve replacement operation. Ann Thorac Surg 2003; 75: 830-834.
- 18. Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999; 15: 816-822.
- 19. Osswald BR, Gegouskov V, Badowski-Zyla D, et al. Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J 2009; 30: 74-80.
- 20. Edwards FH, Grover FL, Shroyer AL, et al. The Society of Thoracic Surgeons National Cardiac Surgery Database: current risk assessment. Ann Thorac Surg 1997; 63: 903-908.
- 21. Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009; 88: 468-474.
- 22. Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009; 250: 449-455.
- 23. Piazza N, Grube E, Gerckens U, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) CoreValve ReValving System: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention 2008; 4: 242-249.
- 24. Otten AM, van Domburg RT, van Gameren M, et al. Population characteristics, treatment assignment and survival of patients with aortic stenosis referred for percutaneous valve replacement. EuroIntervention 2008; 4: 250-255.
- 25. Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47: 1214-1223.
- 26. Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116: 755-763.
- 27. Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 2009; 119: 3009-3016.
- 28. Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50: 69-76.
- 29. Bleiziffer S, Ruge H, Mazzitelli D, et al. 18 months single center experience with the CoreValve prosthesis in 157 patients: procedural complications, valve-related events and survival [abstract]. EuroIntervention 2009; 5 Suppl E: E44.
- 30. Tamburino C, Capodanno D, Mulè M, et al. Procedural success and 30-day clinical outcomes after percutaneous aortic valve replacement using current third-generation self-expanding CoreValve prosthesis. J Invasive Cardiol 2009; 21: 93-98.
- 31. Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007; 116 (11 Suppl): I240-I245.
- 32. Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: 1-year outcome in 26 patients. J Thorac Cardiovasc Surg 2009; 137: 167-173.
- 33. Piazza N, Onuma Y, Jesserun E, et al. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv 2008; 1: 310-316.
- 34. Chiam PTL, Ruiz CE. Percutaneous transcatheter aortic valve implantation: evolution of the technology. Am Heart J 2009; 157: 229-242.
- 35. Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using Corevalve Revalving system. Catheter Cardiovasc Interv 2007; 70: 760-764.





Abstract
As the population ages, the prevalence of aortic stenosis is increasing.
There is an unmet clinical need for the treatment of aortic stenosis in high-risk patients, who are often older, frail and have multiple comorbidities.
Percutaneous aortic valve replacement (PAVR) is a new and innovative technique for the management of high-risk patients with aortic stenosis.
There are currently two devices under evaluation in clinical trials in Australia: the CoreValve ReValving System and the Edwards SAPIEN valve.
These devices are generally deployed retrogradely, mainly transfemorally or via the subclavian artery or, less commonly, transapically.
Initial experience has been encouraging, with good short-term outcomes. However, there is a lack of long-term data.
PAVR is presently only advocated for high-risk older patients with symptomatic aortic stenosis.
Where PAVR lies in the treatment algorithm for aortic stenosis will be determined by randomised controlled trials, but for now it offers a genuine treatment alternative for high-risk patients.