Pancreatic cancer represents 2% of all cancers, but accounts for 5% of all cancer deaths.1 In Victoria during 2006, 580 new cases were diagnosed and 529 people died from pancreatic cancer.1 Median survival after diagnosis is around 3 to 6 months.1,2 Five-year survival is less than 5% and has improved only slightly over the past 20 years.2 Surgery is the only curative treatment and results in long-term survival of less than 20%.3,4 However, most patients present with unresectable disease and many experience rapid clinical deterioration. This has not changed over several decades.5,6
Small trials have shown that survival in patients with locally advanced but unresectable disease may be improved using a combination of chemotherapy and radiotherapy, rather than radiotherapy alone.7-9 Various studies of chemotherapy and radiotherapy suggest that adjuvant therapy may improve outcomes for patients after surgery.10-15 Most patients with pancreatic cancer will develop metastases and are potential candidates for treatment with systemic chemotherapy, which may result in clinical benefit and is associated with a small survival advantage.16
Descriptive statistics were analysed using SPSS 14.0, release 14.0.2 (SPSS, Chicago, Ill, USA).
Ethics approval was obtained from the Cancer Council Victoria Human Research Ethics Committee.
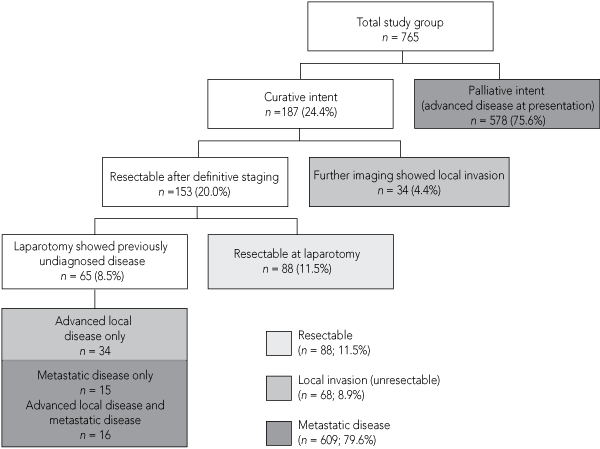
Of 1044 patients identified from the VCR, 927 were eligible for the study. Reasons for ineligibility included incomplete diagnostic information or notification by death certificate only. Completed surveys were obtained for 831 of the 927 eligible patients, a response rate of 89.6%. Patients with tumours of the ampulla of Vater and neuroendocrine tumours were excluded from the analysis, leaving 765 patients. Only 52 patients (6.8%) were managed in a multimodality clinic. Box 1 shows patients by treatment intent and disease stage.
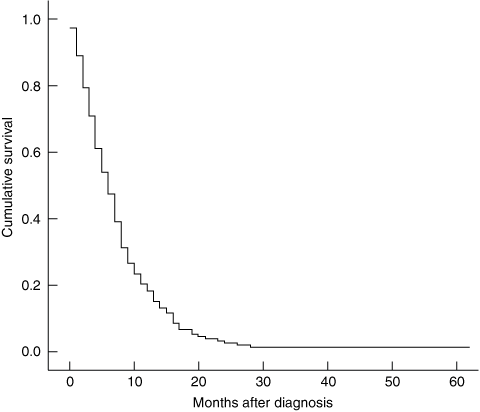
Chemotherapy as palliative treatment. Among the patients treated with chemotherapy as a palliative treatment, single-agent gemcitabine was the most common treatment (154/185; 83.2%). The age of patients who received palliative chemotherapy broadly reflected the age of patients with pancreatic cancer:1 33.5% were 70 years or older (62/185), and 7.6% were 80 years or older (14/185). Duration of treatment varied from 1 day to more than 24 weeks, with 29.2% of patients (54/185) receiving chemotherapy for 6–12 weeks, 28.1% (52/185) for 12–24 weeks and 10.8% (20/185) for more than 24 weeks (unknown duration for 2.7%, 5/185). Treatment was discontinued owing to the planned course being complete (21/185; 11.4%), disease progression (109/185; 58.9%), poor tolerance (24/185; 13.0%), patient choice (11/185; 5.9%), deteriorating general health (10/185; 5.4%), and unspecified reasons (10/185; 5.4%). Median overall survival for patients treated with single-agent gemcitabine was 6.6 months (Box 2).
Of the patients who received radiotherapy and for whom questionnaires were completed (119/765; 15.6% of the study cohort), referrals were mostly from medical oncologists (95; 79.8%), general surgeons (9; 7.6%) and HPB surgeons (5; 4.2%). According to Eastern Cooperative Oncology Group (ECOG) performance status (a scale measuring fitness and wellbeing, where 0 is fully active and able to carry on all pre-disease performance without restriction and 4 is completely disabled and confined to bed or chair), patients who received radiotherapy generally had a reasonable performance status: a score of 0 for 6.7% of patients (8/119), 1 for 35.3% of patients (42/119) and 2 for 15.1% of patients (18/119). The score was unknown for 45 patients (37.8%), 3 for five patients and 4 for one patient. Ten patients who received radiotherapy (8.4%) were treated as part of a clinical trial.
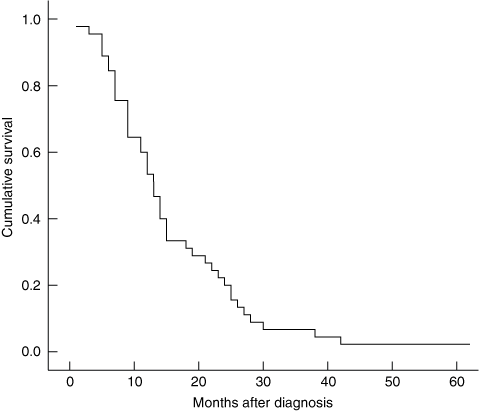
Chemoradiation as radical treatment. Of the 45 patients referred for curative, radical radiotherapy 41 were referred by a medical oncologist. All received concurrent chemotherapy. Nine patients were enrolled in a clinical trial, some of whom received concurrent gemcitabine. Most patients (43/45) underwent three-dimensional computer planning. Patients were generally treated with a three-field or four-field technique (23 and 21 patients, respectively). Forty-three patients were treated with a dose of at least 45 Gy; 25 patients received 50.4 Gy in 28 fractions and 12 received 54 Gy in 30 fractions. Chemoradiation-related toxicity requiring treatment occurred in 13 patients, consisting mainly of nausea and vomiting (11 patients), and seven patients required a break in treatment. Median overall survival for patients who received radical chemoradiation was 13.1 months (Box 3).
To our knowledge, this study is one of few population-based surveys of the management of pancreatic cancer. Clinical management surveys can help promote informed patient choices and may result in development and implementation of standard protocols, identification and reduction of variations in practice, promotion of multidisciplinary care and clinical research, and collection and reporting of standard datasets.17
We found low rates of use of chemotherapy and radiotherapy in patients with pancreatic cancer. Consistent with other studies, including an evaluation of the United States National Cancer Data Base of 100 000 people with pancreatic cancer, a large proportion of people did not have any cancer-directed treatment.6,18 Although some patients were considered too unwell for cancer-directed treatment, for many there were no clear reasons why they were not referred for consideration of such treatment.
Low referral rates to medical and radiation oncologists may be related to general pessimism regarding the impact of these treatments for people with pancreatic cancer. We have previously shown that gastroenterologists and general surgeons appear to be more pessimistic than HPB surgeons and medical and radiation oncologists.19 Consistent with this finding, medical oncologists were the most common referral source for consideration of radiotherapy in our study. Also, in patients who were referred for postoperative radiotherapy, most referrals (20/26) were by a medical oncologist, rather than a surgeon.
Optimal use of chemotherapy and radiotherapy might be improved if all cases of pancreatic cancer were managed in a multidisciplinary setting and discussed in a multidisciplinary team meeting. In this study, a very small proportion of patients were managed in a multimodality clinic. It has been suggested that the increased use of multimodality therapy over time has been associated with a beneficial impact on survival.5 Currently, in Victoria, there is a government-led push for greater use of multidisciplinary care teams, as well as documentation of discussions from multidisciplinary team meetings, including reportable key indicators.
Trials comparing chemoradiation to chemotherapy alone for patients with locally advanced pancreatic cancer have produced conflicting results. Several early small trials suggested a significantly longer survival following chemoradiation compared with chemotherapy or radiotherapy alone.7-9 A recently reported study, in which 119 patients with locally advanced pancreatic cancer were randomly assigned to receive gemcitabine chemotherapy alone or chemoradiation, showed superior overall survival for patients who received gemcitabine chemotherapy alone (median survival, 13.0 months v 8.6 months; P = 0.03). One-year survival was 53% and 32%, respectively, which led to early closure of the trial.20 Recently reported results from an ECOG trial (E4201), in which 74 patients with locally unresectable pancreatic cancer were randomly assigned to receive gemcitabine alone or chemoradiation followed by further gemcitabine, showed that patients in the chemoradiation arm had a higher median survival than patients who received gemcitabine alone (11.0 v 9.2 months; P = 0.04).21
Radical chemoradiation should be considered for patients with locally advanced pancreatic cancer who have good performance status. Patients in this study who received radical chemoradiation had a median overall survival of 13.1 months, consistent with other published findings.7-9 In contrast, results from the primary questionnaire in this study (not yet published) showed that patients undergoing stent insertion or bypass surgery had median overall survival of 3–6 months.
The benefit of postoperative adjuvant therapy was subject to debate in 2002 and 2003 — when the patients in our study were diagnosed — and has remained controversial.10-15 In 1985, it was suggested that median survival was improved with postoperative adjuvant chemoradiation and maintenance 5-fluorouracil chemotherapy, compared with no postoperative adjuvant therapy.13 Another study suggested that postoperative adjuvant therapy provides a survival advantage for patients with carcinoma of the pancreatic head.22 A key trial, reported in 2004, has added to the controversy: the European Study Group for Pancreatic Cancer 1 Trial showed that adjuvant chemotherapy resulted in a significant survival benefit, whereas adjuvant chemoradiotherapy had a deleterious effect on survival.12 This study has been heavily criticised, primarily because of concerns about surgical and radiation therapy quality control and randomisation methodology.
Most patients with pancreatic cancer have metastatic disease at presentation; these patients may gain symptomatic benefit and a small survival advantage from systemic chemotherapy.16 Treatment is generally well tolerated. The pivotal trial of gemcitabine chemotherapy for patients with advanced, unresectable pancreatic cancer showed that 23.8% of patients gained clinical benefit defined as a sustained (> 4 week) improvement in at least one of pain, performance status and weight, without worsening of the others.16 Median overall survival was 5.7 months. Similarly, we showed that gemcitabine chemotherapy, used as a palliative treatment, was well tolerated and associated with a median overall survival of 6.6 months.
1 Treatment intent and surgical resectability for patients diagnosed with pancreatic cancer during 2002–2003 in Victoria
Received 30 July 2009, accepted 22 September 2009
- Michael Jefford1,2,3
- Vicky Thursfield3
- Yvonne Torn-Broers3
- Trevor Leong1
- Mario Guerrieri4
- Tony Speer5
- 1 Peter MacCallum Cancer Centre, Melbourne, VIC.
- 2 University of Melbourne, Melbourne, VIC.
- 3 Cancer Council Victoria, Melbourne, VIC.
- 4 Radiation Oncology Victoria, Melbourne, VIC.
- 5 Royal Melbourne Hospital, Melbourne, VIC.
We acknowledge that Professor John Zalcberg, Peter MacCallum Cancer Centre, originally suggested that the Victorian Cooperative Oncology Group and the VCR conduct a clinical management survey of people with pancreatic cancer.
None identified.
- 1. Thursfield V, Farrugia H, Giles G. Cancer in Victoria 2006. Canstat No. 46. Melbourne: Cancer Council Victoria, 2009.
- 2. English D, Farrugia H, Thursfield V, et al. Cancer survival Victoria 2007. Estimates of survival in 2004 (and comparisons with earlier periods). Melbourne: Cancer Council Victoria, 2007.
- 3. Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59-66.
- 4. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas — 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567-579.
- 5. Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the US: utilization, outcomes, and the effect of hospital volume. Cancer 2007; 110: 1227-1234.
- 6. Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 1999; 189: 1-7.
- 7. Gunderson LL, Martin JK, Kvols LK, et al. Intraoperative and external beam irradiation +/- 5-FU for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 1987; 13: 319-329.
- 8. Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 1988; 80: 751-755.
- 9. Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981; 48: 1705-1710.
- 10. Verslype C, Van Cutsem E, Dicato M, et al. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann Oncol 2007; 18 Suppl 7: vii1-vii10.
- 11. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007; 297: 267-277.
- 12. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200-1210.
- 13. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899-903.
- 14. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999; 230: 776-782; discussion 782-784.
- 15. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008; 299: 1019-1026.
- 16. Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403-2413.
- 17. Staples M, Elwood M, St John J, et al. Perceived impact on clinical practice and logistical issues in clinical management surveys of cancer: Australian experience. Qual Saf Health Care 2009; 18: 195-198.
- 18. Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 2003; 21: 3409-3414.
- 19. Jefford M, Jennens R, Speer T, Thursfield V. Different professionals’ knowledge and perceptions of the management of people with pancreatic cancer. Asia Pac J Clin Oncol 2007; 3: 44-51.
- 20. Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 2008; 19: 1592-1599.
- 21. Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201 [abstract]. J Clin Oncol 2008; 26 (15S): 4506.
- 22. Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg 2006; 244: 332-333; author reply 333.





Abstract
Objective: To describe the management and outcomes of a population-based cohort of patients with pancreatic cancer treated with chemotherapy or radiotherapy in Victoria, Australia.
Design, setting and patients: Questionnaire-based study of patients diagnosed with pancreatic cancer during 2002–2003 in Victoria who were retrospectively identified from the Victorian Cancer Registry and followed up for a minimum of 5 years.
Main outcome measures: Reported treatment, referral patterns and survival rates.
Results: 1044 patients with pancreatic cancer were identified, of whom 927 were eligible for the study. Completed questionnaires were obtained for 831 eligible patients (response rate, 89.6%) and data for 66 patients with tumours of the ampulla of Vater and neuroendocrine tumours were excluded. Of the remaining 765 patients, 6.5% were managed in multimodality clinics. Chemotherapy was considered for 413 patients and radiotherapy was considered for 162. One-third of the cohort (275 patients) received chemotherapy, most commonly as palliative treatment (185). Single-agent gemcitabine was the most common palliative treatment (154), and was associated with a median overall survival of 6.6 months. Radiotherapy was used in 119 patients (15.6% of the cohort) — it was used alone or with chemotherapy, as postoperative adjuvant treatment, as potentially curative radical treatment, or as palliative treatment. For 45 patients with locally advanced disease who were treated with chemoradiation as radical treatment, median overall survival was 13.1 months.
Conclusions: There appears to be under-referral of patients to medical and radiation oncologists. Median survival of patients treated with radical chemoradiation or palliative chemotherapy is consistent with clinical trial data, but outcomes for patients in our cohort were generally poor. Development and implementation of treatment guidelines may result in improved outcomes.