Ethics review of multisite studies involving human participants has long been a challenge for researchers in Australia1-3 and overseas.4,5 During the past decade, a series of reports, primarily from the United States6-13 and United Kingdom,14-16 have documented negative experiences with repeated reviews of the same study by ethics committees at multiple sites. Researchers’ concerns include cost, inefficiency, tardiness, duplication, and the variable and inconsistent nature of ethical concerns identified across committees.
Following the UK’s lead in this area, Australia is moving to address the problem by introducing procedures for a single locus of review for multisite studies that would have authority across all or most sites.17,18 Centralised ethics review is a logical strategy for bringing efficiencies to the oversight of multisite studies. It is relatively easy to envision how this approach will work for some types of research — for example, a clinical trial recruiting patients at tertiary care hospitals in several states. For other types of research, it is less clear. In community-based population health research, for example, moves towards centralisation will force difficult trade-offs that could limit the role of local concerns and community input in the review process.
Multisite Indigenous health research looms as a particularly vexing challenge. Current national ethics guidelines stress the need to scrutinise researchers’ engagement with Indigenous communities; the regulatory goal is to ensure that the design and conduct of the research is sensitive to the interests and concerns of those communities19 (although there remains considerable debate and controversy regarding how best to achieve effective engagement20-23). Centralisation of ethics review of multisite studies will not displace those responsibilities. Hence, the burdens and barriers imposed by ethics review of multisite Indigenous health research could remain substantial.
The NIEHS is a multistage randomised cluster study. It was designed to assess the prevalence and principle causes of vision impairment, the utilisation of eye care services, the barriers to eye health, and the impact of vision impairment among Indigenous Australians. The study methods are summarised in Box 1 and described in detail elsewhere.24
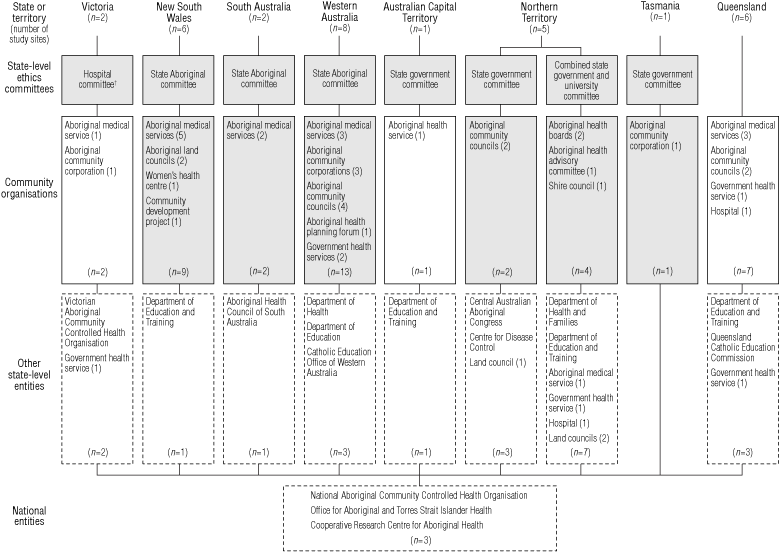
To conduct the NIEHS, we sought ethics approval and engaged in community consultation in all Australian states and territories during 2007 and 2008. Written correspondence relating to these activities was reviewed and analysed (Box 1). An overview of the process of ethics review and consultation for the NIEHS is provided in Box 2. A summary of the NIEHS ethics review experience is described in Box 3 and issues raised during the ethics review are categorised in Box 4.
Before the NIEHS could proceed, it was necessary to work through an elaborate process of ethics review and community consultation. This involved correspondence with 73 entities (Box 2). Only eight of the entities were Human Research Ethics Committees (HRECs) with formal approval authority, but those HRECs operated as gateways to 31 separate community organisations whose sign-off was essentially a pre-condition to ethics approval. The 39 entities with approval authority raised a total of 80 discrete ethics-related issues (Box 4).
Centralised ethics review is a widely discussed policy reform for improving the efficiency of multisite research. The UK has moved aggressively in this direction.25 Specially constituted committees within National Health Service trusts are empowered to confer approvals that operate throughout the UK. Although this reform does not appear to have eliminated the workload associated with gaining approval from local ethics committees, early reports suggest that it has streamlined overall approval processes markedly.26-28
Similar reforms are pending in Australia. Under direction from the Australian Health Ministers’ Advisory Council, the National Health and Medical Research Council (NHMRC) established the Harmonisation of Multi-centre Ethical Review (HoMER) project during early 2007. This project involves development of a nationally harmonised system in which a single ethics and scientific review by an approved committee will cover multiple institutions, within and across states and territories.18 New South Wales and Queensland already operate intrastate versions of such a system, and Victoria is currently developing one.
It is unclear how centralised ethics review will operate in the context of community-based studies, like the NIEHS, in which there are many local stakeholders. Centralisation will not change the desire of those stakeholders to have a say in how research is done in their communities, nor should it. Community organisations bring an important perspective — which an expert group focused on scientific issues and the requirements set forth in the National statement on ethical conduct in human research29 will not have — and the ethics review process is deficient without this perspective.30 In addition, effective community engagement enables effective implementation of research findings.
On the other hand, many layers of ethics approval and consultation almost certainly deter or derail large-scale investigation and surveillance in areas of national importance, such as Indigenous health.31 If our experience with the NIEHS is a reliable guide, the administrative and logistical burdens of such ethics review are substantial. From a population health standpoint, this is an unfortunate outcome. Efforts to “close the gap” in the health of Aboriginal and Torres Strait Islander peoples depend on rigorous clinical and epidemiological research, some of it at the national level. Large-scale studies, arguably more than their small-scale counterparts, have the potential to produce the paradigm shifts in knowledge, public opinion and political will that are needed to make major strides towards improving Indigenous health.
Second, linking community consultation to the HREC process, by making the former a pre-condition of the latter, is problematic. Both are important, but they are qualitatively different steps. The National statement on ethical conduct in human research specifies that research methods be “respectful and acknowledge the cultural distinctiveness of discrete . . . communities or groups” and calls for “evidence of support for the research project from relevant Aboriginal and Torres Strait Islander communities.”29 On the ground, the engagement process involves trust and relationship building, and occurs at several levels. For example, basic courtesy dictates the need for introductions and reasonable notice before researchers “arrive”. Logistical necessities, particularly when local staff are involved or the research intersects with existing services, demand another type of dialogue. Ensuring that community leaders are comfortable with and support the study requires a different form of engagement again — done well, it involves discussions before, during and after the field component of the research.
1 Analysis of the ethics review and community consultation processes associated with the National Indigenous Eye Health Survey (NIEHS)
The NIEHS involved a representative sample of 3000 Indigenous adults and children from 30 sites in all Australian states and territories.
Participants completed a questionnaire on access to and utilisation of eye health services, and then underwent standard eye tests; participants with visual impairment then completed a questionnaire on quality of life.
Data were collected between February and December 2008.
Ethics review and community consultation process
During the planning stages, NIEHS investigators approached state and territory departments of health and Aboriginal community-controlled health organisations to seek advice on where to obtain appropriate ethics approvals to cover study activities in each state and territory.
Appropriate community, state and federal entities to consult with were identified by seeking advice from a variety of sources (eg, ethics committees, community leaders in the study site areas, investigators’ contacts).
Efforts to secure ethics approval and community consultation activities began in February 2007 and were completed in December 2008.
Analysis of ethics review experience
Written correspondence for seeking ethics approval was reviewed to construct two inventories: a list of corresponding entities by state and territory, and a summary of issues raised by each entity.
An “issue” was defined as a query, concern or request that required a response from the NIEHS team; responses to some issues required alterations to the study protocol. In some cases, it was unclear whether matters raised constituted a discrete issue or formed a part of another issue; this was resolved by investigator consensus.
Two of us (T M V and D M S) independently generated a set of issue types, based on a list of the issues raised and standard items covered in ethics review (eg, site selection, recruitment, participant information and consent forms, questionnaire design, risks and benefits). The two typologies were then compared, a final set of 18 types was determined, and the issues were categorised accordingly.
2 Entities involved in ethics review and consultation for the National Indigenous Eye Health Survey (NIEHS)*
 |
3 Summary of the NIEHS ethics review and consultation experience
Ethics approval was required from 39 entities (8 HRECs, 31 community organisations).
Consultations were conducted with 34 additional community, state and federal entities.
Every jurisdiction except Queensland and Victoria required approval by a state-level HREC (or, in the case of Victoria, an HREC with state-level approval authority), and the Northern Territory required two separate state-level approvals.
HREC type varied across jurisdictions: hospital committee (Victoria); state Aboriginal committees (New South Wales, South Australia, Western Australia); state government committees (Australian Capital Territory, Northern Territory, Tasmania).
Consultations were undertaken with 41 community organisations (one or more in every jurisdiction; mean of 5.1 per state or territory).
The most common types were Aboriginal medical services or boards (17) and community councils or corporations (15), which together accounted for 78% of community consultations.
Community organisation consent was a mandatory component of ethics review in six of eight jurisdictions.
For 31 of 41 consultations, community organisation approval was a precondition of formal ethics approval.
Within jurisdictions, the numbers of consultations correlated strongly with the numbers of study sites (r = 0.98, P < 0.001).
Consultations were undertaken with 24 additional entities and organisations (21 state, three federal).
These were suggested by an HREC or community organisation, but voluntarily undertaken (ie, ethics approval did not hinge on it).
A total of 80 issues were raised: 60 by HRECs (mean number of issues per HREC, 7.5 [range, 1–26]) and 20 during community consultation and consent processes.
Issues ranged from relatively minor concerns (eg, requests to supply details of insurance coverage) to substantial concerns requiring alteration of study protocol (eg, revisions to the participant information sheet and questionnaire).
The most common issue types were:
requests to obtain approvals from additional third parties (10)
questions and concerns about benefits of study to participants and local community (8)
requests for amendments to participant information sheet (9)
concerns about arrangements with local study staff (7).
Within issue types, there was a substantial degree of overlap in the specific matters raised — issues were rarely identical, but were often variations on a theme.
Procedural aspects of ethics reviews
One HREC accepted prior approval from another committee; the rest did not.
Seven HRECs accepted the standardised National Ethics Application Form (available at <https://www.neaf.gov.au/default.aspx>).
Six HRECs required annual progress reports and two required 6-monthly progress reports.
Six HRECs had different approval periods (ranging from 1 to 5 years) and the approval period was unspecified for two HRECs.*
One HREC stipulated that manuscripts reporting findings from the NIEHS must be reviewed and approved by it before publication.
4 Issues raised during ethics review of the National Indigenous Eye Health Survey, by jurisdiction and type of issue*
Received 29 July 2009, accepted 5 October 2009
- David M Studdert1
- Tamara M Vu1
- Sarah S Fox2,3
- Ian P Anderson1
- Jill E Keeffe2,3,4
- Hugh R Taylor1,4
- 1 Melbourne School of Population Health, University of Melbourne, Melbourne, VIC.
- 2 Centre for Eye Research Australia, Melbourne, VIC.
- 3 Royal Victorian Eye and Ear Hospital, Melbourne, VIC.
- 4 Vision Cooperative Research Centre, Sydney, NSW.
Michelle Mello provided helpful comments on an earlier draft of the manuscript.
None identified.
- 1. Weeramanthri T, Currie BJ. Isn’t one institutional ethics committee’s approval enough? Med J Aust 1994; 161: 398-399.
- 2. Banscott Health Consulting. Report of the review of access to unapproved therapeutic goods. Canberra: Therapeutic Goods Administration, 2005. http://www.tga.gov.au/consult/2005/cltrialrevrep.pdf (accessed Jun 2009).
- 3. Jamrozik K, Kolybaba M. Are ethics committees retarding the improvement of health services in Australia? Med J Aust 1999; 170: 26-28. <MJA full text>
- 4. Gold JL, Dewa CS. Institutional review boards and multisite studies in health services research: is there a better way? Health Serv Res 2005; 40: 291-308.
- 5. Christian MC, Goldberg JL, Killen J, et al. A central institutional review board for multi institutional trials. N Engl J Med 2002; 346: 1405-1408.
- 6. Dziak K, Anderson R, Sevick MA, et al. Variations among institutional review board reviews in a multisite health services research study. Health Serv Res 2005; 40: 279-290.
- 7. Vick CC, Finan KR, Kiefe C, et al. Variation in institutional review processes for a multisite observational study. Am J Surg 2005; 190: 805-809.
- 8. Green LA, Lowery JC, Kowalski CP, Wyszewianski L. Impact of institutional review board practice variation on observational health services research. Health Serv Res 2006; 41: 214-231.
- 9. Larson E, Bratts T, Zwanziger J, Stone P. A survey of IRB process in 68 US hospitals. J Nurs Scholarsh 2004; 36: 260-265.
- 10. Dyrbye LN, Thomas MR, Mechaber AJ, et al. Medical education research and IRB review: an analysis and comparison of the IRB review process at six institutions. Acad Med 2007; 82: 654-660.
- 11. Silverman H, Hull SC, Sugarman J. Variability among institutional review boards’ decisions within the context of a multicenter trial. Crit Care Med 2001; 29: 235-241.
- 12. Burman W, Breese P, Weis S, et al. The effects of a local review on informed consent documents from a multicenter clinical trials consortium. Control Clin Trials 2003; 24: 245-255.
- 13. McWilliams R, Hoover-Fong J, Hamosh A, et al. Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA 2003; 290: 360-366.
- 14. Al-Shahi Salman R, Brock TM, Dennis MS, et al. Research governance impediments to clinical trials: a retrospective survey. J R Soc Med 2007; 100: 101-104.
- 15. Ah-See KW, Mackenzie J, Thakker NS, Maran AGD. Local research ethics committee approval for a national study in Scotland. J R Coll Surg Edinb 1998; 43: 303-305.
- 16. Middle C, Johnson A, Petty T, et al. Ethics approval for a national postal survey: recent experience. BMJ 1995; 311: 659-660.
- 17. Jenkin R, Bennett J, Frommer M, Madronio C. A streamlined national approach to scientific and ethics review of multi-centre health and medical research in Australia: issues and options, 2006. http://www.nhmrc.gov.au/_files_nhmrc/file/health_ethics/hrecs/A_streamlined_national_approach_to%20scientific_and_ethics_reviewvof%20multi-centre_health_and_medical_research_in_Australia.pdf (accessed Jan 2010).
- 18. National Health and Medical Research Council. Harmonisation of Multi-centre Ethical Review (HoMER) enabling system: proposed national approach for the adoption of a single ethical and scientific review for multi-centre health and medical human research. Canberra: NHMRC, 2008. http://www.nhmrc.gov.au/_files_nhmrc/file/health_ethics/homer/consultation/homer-consultation-complete.pdf (accessed Jan 2010).
- 19. National Health and Medical Research Council. Values and ethics: guidelines for ethical conduct in Aboriginal and Torres Strait Islander research. Canberra: NHMRC, 2003. http://www.nhmrc.gov.au/_files_nhmrc/file/health_ethics/human/conduct/guidelines/e52.pdf (accessed Jan 2010).
- 20. Anderson I. Ethics and health research in Aboriginal communities. In: Daly J, editor. Ethical intersections: health research, methods and researcher responsibility. Sydney: Allen and Unwin, 1996.
- 21. Griew RM, McAulley D. Review of the interim guidelines on ethical matters in Aboriginal and Torres Strait Islander health: background paper. Canberra: Australian Health Ethics Committee, 2001.
- 22. Anderson I, Griew R, McAullay D. Ethics guidelines, health research and Indigenous Australians. N Z Bioeth J 2003; 4: 20-29.
- 23. Humphery K. The development of the National Health and Medical Research Council guidelines on ethical matters in Aboriginal and Torres Strait Islander health research: a brief documentary and oral history. Melbourne: VicHealth Koori Health Research and Community Development Unit, University of Melbourne, 2003. (Discussion Paper No. 8.)
- 24. National Indigenous Eye Health Survey Team. National Indigenous Eye Health Survey: full report. Melbourne: University of Melbourne, 2009. http://www.cera.org.au/publications/reports/091012%20NIEHS %20Final%20Report%20V2_0d.pdf (accessed Jan 2010).
- 25. Central Office for Research Ethics Committees. Governance arrangements for NHS research ethics committees. London: COREC, 2001. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4005727 (accessed Jun 2009).
- 26. Dunn NR, Arscott A, Mann RD. Costs of seeking ethics approval before and after the introduction of multicenter research ethics committees. J Roy Soc Med 2000; 93: 511-512.
- 27. Tully J, Ninis N, Booy R, Viner R. The new system of review by multicenter research ethics committees: Prospective study. BMJ 2000; 320: 1179-1182.
- 28. Al-Shahi R. Research ethics committees in the UK — the pressure is now on research and development departments. J Roy Soc Med 2005; 98: 444-447.
- 29. National Health and Medical Research Council. National statement on ethical conduct in human research. Canberra: NHMRC, 2007. http://www.nhmrc.gov.au/publications/synopses/e72syn.htm (accessed Jun 2009).
- 30. National Health and Medical Research Council. Keeping research on track: a guide for Aboriginal and Torres Strait Islander peoples about health research ethics. Canberra: NHMRC, 2005. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/e65.pdf (accessed Jun 2009).
- 31. Taylor HR, Fox SS. Ethical hurdles in Indigenous research. Aust N Z J Public Health 2008; 32: 489-490.





Abstract
Researchers have longstanding concerns about the logistical and administrative burdens posed by ethics review of multisite studies involving human participants.
Centralised ethics review, in which approval by one committee has authority across multiple sites, is widely touted as a strategy for streamlining the process. The Harmonisation of Multi-centre Ethical Review (HoMER) project is currently developing such a system for Australia.
It is unclear how centralised review will work for multisite Indigenous health research, where the views of local stakeholders are important and community consultation is mandatory.
Our recent experience in conducting the National Indigenous Eye Health Survey (NIEHS) shows how elaborate the current ethics approval and community consultation processes can be, and points to several lessons and ideas to guide pending reforms.