McKittrick–Wheelock syndrome is a rare but recognised complication of hypersecretory rectosigmoid villous adenoma. Fluid and electrolyte imbalances require close monitoring because of large-volume losses of water, sodium and potassium. We report an unusual presentation of the syndrome associated with the development of acute pseudo-obstruction of the colon, presumably due to electrolyte dysfunction and acute renal failure. (MJA 2010; 192: 225-227)
Initial laboratory investigations (Box 1) showed hyponatraemia, hypokalaemia and elevated urea and creatinine levels. Haemoconcentration was evident. Results of investigations for intrarenal causes of renal failure were negative. Analysis of arterial blood gases showed a transient respiratory alkalosis and net mild metabolic acidosis. There were multiple causative factors, suggesting the presence of an underlying metabolic alkalosis.
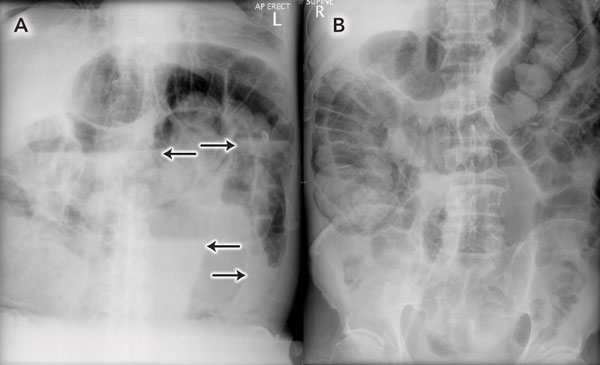
Renal tract ultrasound showed no obstructing lesion, with normal kidney size and morphology. Plain imaging (Box 2) and computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast demonstrated distension of the small bowel up to 4 cm in diameter, with a caecal diameter of 10 cm. Fluid material filled the sigmoid colon and rectum. A non-obstructing, exophytic mass arising from the lateral wall of the rectum, measuring 5 × 9 × 7 cm, was noted. A presumptive diagnosis of colonic pseudo-obstruction due to fluid and electrolyte imbalance was made.
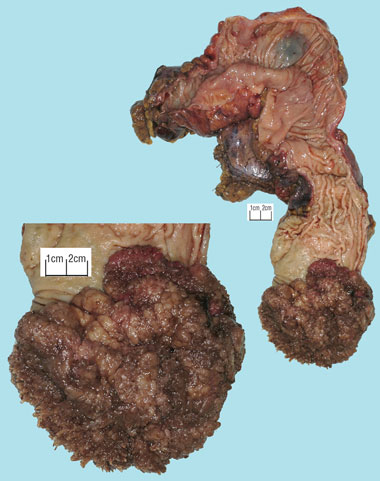
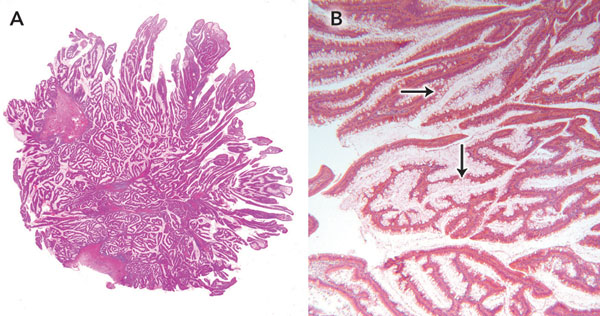
Staging magnetic resonance imaging of the pelvis performed on the day of discharge clearly demonstrated a large, sessile tumour arising from the left rectal wall (Box 3). Appearances were thought to be consistent with a T2 lesion. After 3 weeks of outpatient convalescence, the patient returned for elective low anterior resection, which revealed a large, exophytic lesion within the rectum (Box 4). Histopathological examination of the resected specimen (Box 5) confirmed hypersecretory tubulovillous adenoma with low-grade dysplasia.
McKittrick–Wheelock syndrome was described in 1954 and is a rare complication of villous adenoma.1 It is typified by large-volume secretory diarrhoea, prerenal acute renal failure, and severe electrolyte dysfunction (primarily hyponatraemia, hypochloraemia, hypokalaemia and metabolic acidosis). The causative lesion is usually in the rectosigmoid and is normally over 4 cm in diameter.2 One series of 18 patients described tumours ranging between 7 cm and 18 cm.3 Roughly 2% of patients with rectosigmoid villous adenoma will develop hypersecretory complications.4
Cellular composition of non-secretory and secretory villous adenomas differs markedly. Light microscopy of non-secretory villous adenoma reveals relatively few goblet cells within the tumour epithelium, while much of the epithelial architecture of secretory adenomas is composed of these mucin-secreting structures. It has been postulated that the large surface area of the lesion participating in mucin production is a contributing factor to the volume of diarrhoea. The distal location of these lesions means there is a minimal area of normal colonic mucosa remaining to allow fluid absorption.5
The electrolyte composition of mucin secreted by abnormal cells within the causative lesion is of interest. Whereas normal bowel absorbs sodium and water and secretes potassium, segments of intestine affected by villous adenoma have been found to secrete water, sodium and potassium. Absorptive capacity was largely unchanged. In both cases, net movement of water was directly related to net movement of sodium. There was no relationship between net movement of water and potassium loss.6
The cause of this abnormal secretory function has been postulated to be secretagogue-mediated. Rectal effluent from a patient with villous adenoma of the rectum demonstrated prostaglandin E2 (PGE2) levels three to six times higher than normal.7 Tissue from villous adenoma and carcinoma synthesises more PGE2 than normal colonic mucosa. Mucosa adjacent to adenomatous polyps has been found to be unaffected by this, but carcinoma-associated mucosa was demonstrated to synthesise larger amounts of PGE2.8 These secretagogues are active at sites containing the prostaglandin synthetic pathway. Non-reversible cyclooxygenase-inhibiting agents have been used to reduce PGE2 production, and consequently loss of sodium and water through the rectum.9 Cyclic nucleotides have also been implicated.10 It has previously been suggested that elevated PGE2 levels may contribute to the adenoma–carcinoma sequence,11 explaining the beneficial role of non-steroidal anti-inflammatory drugs in preventing colorectal cancer as well as controlling diarrhoea.
The presence of colonic pseudo-obstruction added another level of complexity to our case.12 Given the pathophysiology of hypersecretory villous adenoma and the predisposing factors for pseudo-obstruction, this association is not unexpected. Less than 5% of patients will develop colonic pseudo-obstruction idiopathically.13 In an analysis of 378 patients with pseudo-obstruction, 16 cases (4.23%) were precipitated by acute renal failure, and a further 15 (3.97%) by electrolyte dysfunction.14 In another retrospective analysis of 48 cases, 83% of patients were found to have some degree of electrolyte dysfunction.15 The mechanism by which homeostatic derangement causes pseudo-obstruction remains poorly understood.
1 Relevant admission laboratory results*
* Some initial volume resuscitation with normal saline had taken place prior to investigations. |
|||||||||||||||
2 Erect (A) and supine (B) abdominal x-rays taken on admission
 |
|
Note the uniform dilation of the large bowel, with multiple air–fluid levels (arrows). |
3 Sagittal (A) and coronal (B) magnetic resonance images of the causative lesion
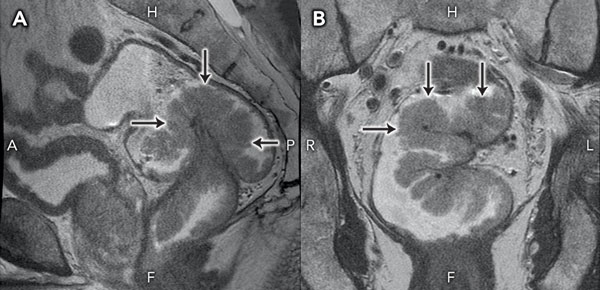 |
H = head. F = feet. A = anterior. P = posterior. R = right. L = left. |
4 Macroscopic photograph of rectal villous adenoma found at operation
 |
|
The causative lesion was 12 cm in diameter, with elevation of 5 cm. |
- 1. McKittrick LS, Wheelock FC Jr. Carcinoma of the colon. Springfield, Ill: Charles C Thomas, 1954: 61.
- 2. Popescu A, Orban-Schiopu A, Becheanu G, Diculescu M. McKittrick–Wheelock syndrome — a rare cause of acute renal failure. Rom J Gastroenterol 2005; 14: 63-66.
- 3. Snhitka T, Friedman MHW, Kidd E, Mackenzie W. Villous tumors of the rectum and colon characterized by severe fluid and electrolyte loss. Surg Gynecol Obstet 1961; 112: 609-621.
- 4. Pheils MT. Villous tumors of the rectum. Dis Colon Rectum 1979; 22: 406-407.
- 5. Older J, Older P, Colker J, Brown R. Secretory villous adenomas that cause depletion syndrome. Arch Intern Med 1999; 159: 879-880.
- 6. Duthie HL, Atwell JD. The absorption of water, sodium and potassium in the large intestine with particular reference to the effects of villous papillomas. Gut 1963; 4: 373-377.
- 7. Steven K, Lange P, Bukhave K, Rask-Madsen J. Prostaglandin E2-mediated secretory diarrhea in villous adenoma of rectum: effect of treatment with indomethacin. Gastroenterology 1981; 80: 1562-1566.
- 8. Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut 1994; 35: 675-678.
- 9. Smelt AH, Meinders AE, Hoekman K, et al. Secretory diarrhea in villous adenoma of rectum: effect of treatment with somatostatin and indomethacin. Prostaglandins 1992; 43: 567-572.
- 10. Jacob H, Schlondorff D, St Onge G, Bernstein LH. Villous adenoma depletion syndrome. Evidence for a cyclic nucleotide-mediated diarrhea. Dig Dis Sci 1985; 30: 637-641.
- 11. Rosenberg L, Palmer JR, Zauber AG, et al. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst 1991; 83: 355-358.
- 12. Ogilvie H. Large-intestine colic due to sympathetic deprivation; a new clinical syndrome. Br Med J 1948; 2: 671-673.
- 13. Saunders MD. Acute colonic pseudo-obstruction. Gastrointest Endosc Clin N Am 2007; 17: 341-360.
- 14. Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis Colon Rectum 1986; 29: 203-210.
- 15. Jetmore AB, Timmcke AE, Gathright JB Jr, et al. Ogilvie’s syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum 1992; 35: 1135-1142.





Special thanks to Dr Vincent Pellegrino from the Alfred Intensive Care Unit for his assistance in interpreting the initial acid–base picture, and Julian Richardson from the histopathology department at Cabrini Hospital for the macroscopic and microscopic photography of the surgical specimen.
None identified.