Internationally, recommendations and practice differ regarding choice of agent and duration of administration of prophylactic antibiotics for cardiac surgery.1 The current Australian Therapeutic guidelines: antibiotic (version 13) recommend either cephalothin or cephazolin or the co-administration of flucloxacillin and gentamicin at the time of induction of anaesthesia before surgery (Box 1).2 Notably, the guidelines recommend against ongoing administration of antibiotic agents after completion of surgery. Although routine use of vancomycin is discouraged, this important agent is recommended for patients likely to be infected or colonised with methicillin-resistant Staphylococcus aureus (MRSA), as well as for patients requiring cardiac valve reoperation (return to theatre or revision) or in patients hypersensitive to penicillins or cephalosporins.
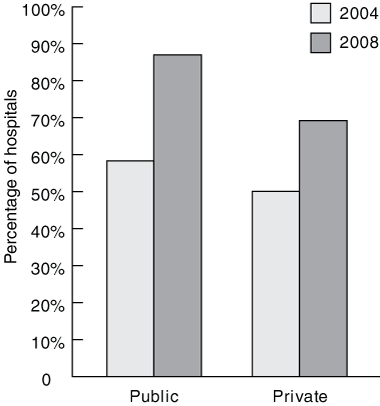
Across all units, an antibiotic protocol was reportedly used for cardiac surgery patients in 13/26 private hospital units (50%) and 14/24 public hospital units (58%) in 2004, compared with 18/26 (69%) and 20/23 (87%), respectively, in 2008 (Box 2). In paired comparisons, protocol use increased from 26/45 units (58%) in 2004 to 36/45 (80%) in 2008 (P = 0.02, McNemar’s test). Of the 36 units using protocols in 2008, 13 were not in 2004. Three units that were using protocols in 2004 no longer were in 2008.
The numbers of units using categories of prophylactic antibiotics in 2004 and 2008 are shown in Box 3. Routine antibiotic prophylaxis consisted exclusively of a first-generation cephalosporin or the co-administration of flucloxacillin and gentamicin for coronary artery graft surgery (CAGS) in 34/50 units (68%) in 2004 and 29/49 (59%) in 2008 (data not shown). Antimicrobial agent administration was more variable for valve surgery (with or without coronary grafting), with the same combination used for this purpose by 21/50 units (42%) in 2004 and 21/49 (43%) in 2008.
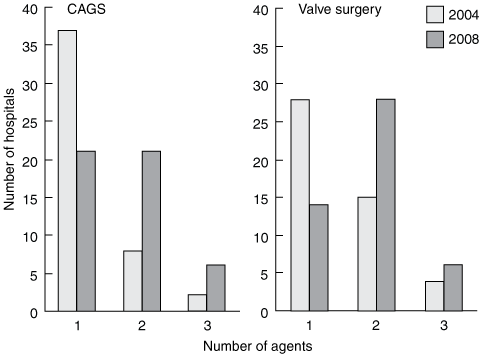
In 2004, the most common practice was use of a single agent for prophylaxis. By contrast, an increase in the use of two or three agents in combination was noted in 2008 (Box 4).
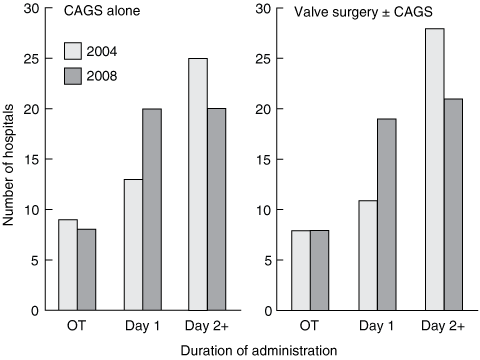
The duration of antibiotic dosing is summarised in Box 5. Surgical units that described continuation of antibiotics until removal of surgical drains rather than to a specific time were included in the count at postoperative Day 1 (24 h), and units continuing antibiotics until removal of intravascular catheters were included in the count at Day 2 (48 h).
A notable change over time, shown in Box 3, was an increased frequency of administration of glycopeptides. While teicoplanin use remained low (two units in 2004, and one in 2008), vancomycin use as part of CAGS prophylaxis increased from 6/45 units (13%) in 2004 to 20/45 units (44%) in 2008 (P < 0.001, exact McNemar’s test). Of the 20 sites using vancomycin for CAGS in 2008, 15 were not in 2004. This vancomycin usage, considered in relation to antibiotic prescribing guidelines, showed evidence of a shift in overall prescription patterns (P = 0.002, exact symmetry test). The largest contribution to this shift in usage was due to 10/45 units (22%) changing from no vancomycin use in 2004 to vancomycin use that was classified as “outside guidelines” in 2008, while only one surgical unit changed from use outside guidelines to no use of vancomycin.
Concordance with the current Australian Therapeutic guidelines: antibiotic recommendation2 for antibiotic prophylaxis in cardiac surgery was found to be low, especially in the context of valve surgery, mainly due to the practice of ongoing postoperative administration of antibiotics in the intensive care unit, the prevalence of which appeared stable over the 4-year period sampled.
Numerous international studies in cardiac surgery have demonstrated that it is relatively common for timing, selection or duration of administration of antimicrobial prophylaxis to be judged as inappropriate or excessive when compared with local guidelines.3-7 Recent Australian research reported a level of 73% concordance with national guidelines for cardiac surgical antibiotic prophylaxis for drug choice, and 45% concordance with guidelines for both drug choice and timing.4
Limitations of clinical practice guidelines include the potential for bias when based on expert opinion in the absence of randomised controlled trials, and inadequate flexibility in the presence of complex interpatient variability. However, full compliance with surgical antibiotic prophylaxis guidelines appears to be associated with low rates of postoperative infectious complications.8 Notably, data from the Victorian Hospital Acquired Infection Surveillance System (VICNISS) seem to indicate stable low rates of cardiac surgical site infections in Victoria over the 4-year period examined in our survey: surgical site infection rates remained around 5% despite apparent differences in antibiotic prophylaxis regimens between cardiac surgical units (unpublished data, VICNISS Coordinating Centre, Melbourne, Vic, 2009).
1 Australian guidelines for antibiotic prophylaxis in cardiac surgery2
Cephalothin 2 g (child: 50 mg/kg up to 2 g) intravenously, at the time of induction of anaesthesia
Di/flucloxacillin 2 g (child: 50 mg/kg up to 2 g) intravenously, at the time of induction
Gentamicin 2 mg/kg intravenously, at the time of induction.
- Timothy P Haydon1,2
- Jeffrey J Presneill3,4
- Megan S Robertson5
- 1 St Vincent’s Hospital, Melbourne, VIC.
- 2 Royal Melbourne Hospital, Melbourne, VIC.
- 3 Mater Health Services, Brisbane, QLD.
- 4 School of Medicine, University of Queensland, Brisbane, QLD.
- 5 Epworth Healthcare, Melbourne, VIC.
We would like to acknowledge the VICNISS Coordinating Centre and participating hospitals for contributing recent unpublished data regarding Victorian surgical site infection rates.
None identified.
- 1. Engelman R, Shahian D, Shemin R, et al. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part II: antibiotic choice. Ann Thorac Surg 2007; 83: 1569-1576.
- 2. Antibiotic Expert Group. Therapeutic guidelines: antibiotic, version 13. Melbourne: Therapeutic Guidelines Limited, 2006.
- 3. Paul M, Porat E, Raz A, et al. Duration of antibiotic prophylaxis for cardiac surgery: prospective observational study. J Infect 2009; 58: 291-298.
- 4. Bull AL, Russo PL, Friedman ND, et al. Compliance with surgical antibiotic prophylaxis — reporting from a statewide surveillance programme in Victoria, Australia. J Hosp Infect 2006; 63: 140-147.
- 5. Kendall JB, Hart CA, Pennefather SH, Russell GN. Infection control measures for adult cardiac surgery in the UK — a survey of current practice. J Hosp Infect 2003; 54: 174-178.
- 6. Pan A, Ambrosini L, Patroni A, et al; Gruppo Italiano di Studio sulle Infezioni in Cardiochirurgia (GIS-InCard) Study Group. Adherence to surgical site infection guidelines in Italian cardiac surgery units. Infection 2009; 37: 148-152.
- 7. Bratzler DW, Houck PM, Richards C, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg 2005; 140: 174-182.
- 8. Miliani KY, L’Hériteau F, Astagneau P. Non-compliance with guidelines for surgical antibiotic prophylaxis and the risk of surgical site infection: results from the INCISO Surveillance Network [abstract]. In: Proceedings of the 19th European Congress of Clinical Microbiology and Infectious Diseases; 2009 May 16–19; Helsinki, Finland. http://www.blackwellpublishing.com/eccmid19/abstract.asp?id=74353 (accessed Dec 2009).





Abstract
Objective: To evaluate national practice for antibiotic prophylaxis in cardiac surgery with respect to the use of protocols, agent selection and duration of administration.
Design, setting and participants: Two point-prevalence surveys of intensive care units in 24 public and 27 private hospitals performing cardiac surgery in Australia, conducted in 2004 and 2008, using a structured telephone questionnaire of the attending senior intensive care clinician in each unit.
Main outcome measures: Existence of a protocol in the unit for antibiotic prophylaxis, specific antibiotic agents used and their duration of administration.
Results: Between 2004 and 2008, reported protocol use increased from 58% to 80% (P = 0.02), while concordance with version 13 of the Australian Therapeutic guidelines: antibiotic for both choice of agent and timing (duration of administration) remained around 10%. Use of multiple agents was common, as was continued antibiotic administration after completion of surgery. Over 4 years, the proportion of cardiac surgical units reporting vancomycin administration for routine valve surgery prophylaxis doubled to 62% (P < 0.001).
Conclusion: Despite an increase in reported protocol use for antibiotic prophylaxis in cardiac surgery, concordance with national antibiotic guidelines remained low, with duration of antibiotic administration deviating most from recommendations. Prophylactic vancomycin use appears to have increased substantially in recent years. Clinical implementation of recommended perioperative cardiac surgical antibiotic prophylaxis may not occur until supported by evidence from either a large prospective randomised study or standardised national surveillance of cardiac surgical site infection rates.