Excessive gestational weight gain has been shown to be associated with higher rates of caesarean delivery, failed induction, instrumental delivery, pre-eclampsia and gestational diabetes mellitus. For the neonate, it increases the incidence of hypoglycaemia, hyperbilirubinaemia, high birthweight, and infant obesity.1-5 Excess gestational weight gain is also associated with postpartum weight retention up to 10 years after pregnancy.6 Unfortunately, excessive weight gain during pregnancy is common, particularly among women who are overweight before pregnancy.7,8 A study in the United States found that 37% of normal weight women and 64% of overweight women experienced excessive gestational weight gain.8
In 1990, the US Institute of Medicine (IOM) published gestational weight-gain guidelines based on body mass index (BMI) before pregnancy (Box 1).9 These guidelines have been widely adopted in clinical practice and are supported by studies showing that weight gain within these guidelines is associated with optimal pregnancy outcomes.1,5,7,10
Few studies have examined measures that may aid women in appropriate gestational weight control and none have examined a simple intervention of regular self-weighing.8,11-15 Outside of pregnancy, the value of frequent self-weighing has been demonstrated.16
The Royal College of Obstetricians and Gynaecologists (London) recommends that, in clinical practice, maternal weight should not be routinely measured during pregnancy. They caution that frequent weighing and feedback may cause undue anxiety among women, with no additional benefit.17 However, this has been refuted for adults who are not pregnant.18
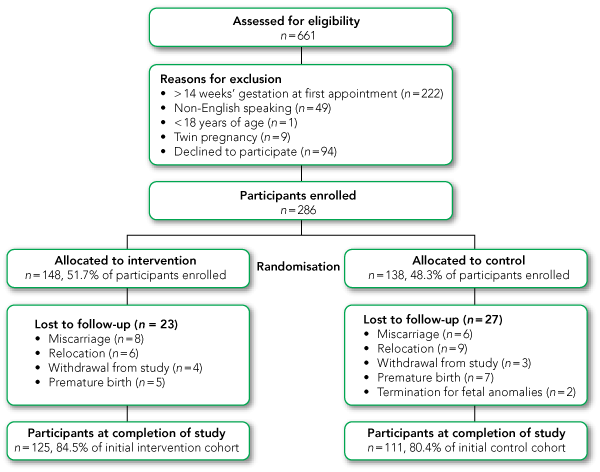
The randomisation sequence was obtained using a computer random number generator. Blocking (which is used to ensure that comparison groups will be of approximately the same size) was not used. Numbered cards allocating women to either the intervention or control group were placed in opaque, sequentially numbered envelopes: 138 women were allocated to the control group and 148 women were allocated to the intervention group. The person generating the allocation sequence was also responsible for participant recruitment; however, allocation concealment was maintained.
Women assigned to the intervention group were given an optimal gestational weight-gain range for their pregnancy, defined by their BMI and the IOM guidelines for weight gain during pregnancy.9 This ideal weight range, together with their weight as measured at recruitment, was recorded on a personalised weight-measurement card (Box 2). Participants were told to record their own weight at 16, 20, 24, 28, 30, 32 and 34 weeks’ gestation, using either a tabular or graphical format provided on the measurement cards. Weight measurements during pregnancy were done on either the participants’ own scales at home or those at the hospital, according to patient preference. Women in the control group were weighed at recruitment and at 36 weeks’ gestation, but were not given any further advice regarding optimal weight gain or regular weighing.
Data are presented as mean (SD), median (25th–75th percentile) or number (%) according to distribution. The primary outcome was weight gain per week of observation; secondary outcomes were the proportion of women exceeding the IOM guidelines, and pregnancy outcomes. For obese women, the IOM recommends a weight gain of 6.8 kg or above. We considered obese women who exceeded 11.5 kg to be above the IOM guidelines, based on the upper limit assigned to overweight women.9
Recruitment took place from July to October 2007. Of the 661 women approached, 281 women were excluded from the study (the reasons are given in Box 3), and 94 women declined to participate (concerns about time and convenience, anxiety about pregnancy, issues about their diet and weight, and plans to deliver at another institution). Of the 286 participants enrolled, 236 completed the study. Those women who were lost to follow-up (Box 3) were not weighed at 36 weeks’ gestation and excluded from the analysis. Participants excluded from the analysis were similar in weight, BMI, age, parity and socioeconomic status to those who completed the study (data not shown).
Baseline characteristics and BMI distribution of the participants are shown in Box 4. There were no clinically meaningful differences between the women in the control and intervention groups in terms of demographic characteristics — age, smoking status, parity, marital status or educational attainment. The ranges of gestational age at recruitment and follow-up were 7.1–14.8 weeks and 36.3–38.3 weeks, respectively.
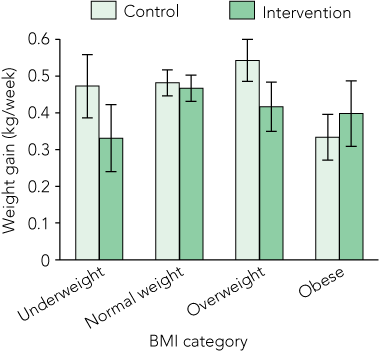
For participants classified as underweight, normal or obese, there was no significant difference in weight gain between intervention and control groups (Box 5). Weight gain for each BMI category is presented in Box 5 and Box 6. The number of women gaining more weight than the IOM-recommended amount was 26/111 (23%) in the control group compared with 23/125 (18%) in the intervention group (Fisher’s exact test, P = 0.42) (Box 5).
In the total study population, there was a trend towards less weight gain in women in the intervention group in all BMI subgroups, except for the obese group (those with a BMI > 29 kg/m2). Women with a BMI > 29 kg/m2 before pregnancy were told to gain at least 6.8 kg, but, in accordance with IOM recommendations, these women were not given an upper weight-gain limit, and this advice may explain the lack of effect in this group.9 A concerning finding was that in the small group of underweight women in our study, there was a non-statistically significant trend towards gaining less weight than the IOM guidelines (P = 0.06; adjusted for multiple comparisons, P = 0.01).
There are two other published randomised controlled trials of interventions to reduce gestational weight gain, both of which showed reduction in some subgroups. These studies included intensive diet and exercise counselling throughout pregnancy and neither were adequately powered (120 and 50 participants, respectively) to demonstrate differences in obstetric or neonatal outcomes.8,15
The limitations of our study include the timing of the first and final weight measurements. The total weight gain in our study was calculated from early pregnancy (≤ 14 weeks’ gestation) until 36 weeks’ gestation. Little weight is gained before 12 weeks’ or after 36 weeks’ gestation, but ideally recruitments should have been before pregnancy, and follow-up continued until labour.19 Our study was also limited by inadequate power to demonstrate differences in obstetric and neonatal outcomes. Moreover, although our finding of reduced weight gain in overweight women in the intervention group reached statistical significance, this result needs to be interpreted with caution, as it was not a pre-specified endpoint for which the study was appropriately powered.
We did not assess the emotional effect that routine weight measurement had on women during pregnancy, or the effect of self-weighing versus weighing by a health professional. Although weight measurement has been shown to have no impact on depressive symptoms in the general population,18 this needs to be further assessed in pregnant women.
5 Gestational weight gain within body mass index (BMI) categories
Proportion gaining more weight than IOM guidelines† |
|||||||||||||||
6 Weight gain per week (95% CI) in the control and intervention groups by body mass index (BMI) category*
 | |||||||||||||||
Received 19 February 2009, accepted 6 August 2009
- Kirby Jeffries1
- Alexis Shub1,2
- Susan P Walker1,2
- Richard Hiscock2
- Michael Permezel1,2
- 1 Department of Obstetrics and Gynaecology, University of Melbourne, Melbourne, VIC.
- 2 Department of Perinatology, Mercy Hospital for Women, Melbourne, VIC.
We would like to thank the women who participated in the study and the staff at the Mercy Hospital for Women for their assistance.
None identified.
- 1. DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol 2007; 110: 745-751.
- 2. Hedderson MM, Weiss NS, Sacks DA, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstet Gynecol 2006; 108: 1153-1161.
- 3. Oken E, Taveras EM, Kleinman KP, et al. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007; 196: 322.e1-e8.
- 4. Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstet Gynecol 2006; 108: 635-643.
- 5. Thorsdottir I, Torfadottir JE, Birgisdottir BE, Geirsson RT. Weight gain in women of normal weight before pregnancy: complications in pregnancy or delivery and birth outcome. Obstet Gynecol 2002; 99: 799-806.
- 6. Rooney BL, Shauberger W. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002; 100: 245-252.
- 7. Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. Am J Public Health 1993; 83: 1100-1103.
- 8. Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnancy. Int J Obes Relat Metab Disord 2002; 26: 1494-1502.
- 9. Institute of Medicine. Committee on Nutritional Status during Pregnancy and Lactation. Nutrition during pregnancy. Part I: weight gain. Part II: nutrient supplements. Washington, DC: National Academy of Sciences, 1990.
- 10. Parker JD, Abrams B. Prenatal weight gain advice: an examination of the recent prenatal weight gain recommendations of the Institute of Medicine. Obstet Gynecol 1992; 79: 664-669.
- 11. Claesson IM, Sydsjö G, Brynhildsen J, et al. Weight gain restriction for obese pregnant women: a case-control intervention study. BJOG 2008; 115: 44-50.
- 12. Gray-Donald K, Robinson E, Collier A, et al. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ 2000; 163: 1247-1251.
- 13. Kinnunen TI, Pasanen M, Aittasalo M, et al. Preventing excessive weight gain during pregnancy — a controlled trial in primary health care. Eur J Clin Nutr 2007; 61: 884-891.
- 14. Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol 2004; 191: 530-536.
- 15. Wolff S, Legarth J, Vangsgaard K, et al. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008; 32: 495-501.
- 16. Linde JA, Jeffery RW, French SA, et al. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med 2005; 30: 210-216.
- 17. National Collaborating Centre for Women’s and Children’s Health. Antenatal care: routine care for the healthy pregnant woman. Clinical examination of pregnant women. London: Royal College of Obstetricians and Gynaecologists, 2003.
- 18. Wing RR, Tate DF, Gorin AA, et al. STOP regain: are there negative effects of daily weighing? J Consult Clin Psychol 2007; 75: 652-656.
- 19. Dawes MG, Grudzinskas JG. Patterns of maternal weight gain in pregnancy. Br J Obstet Gynaecol 1991; 98: 195-201.





Abstract
Objective: To determine if regular weight measurement throughout pregnancy can reduce excessive gestational weight gain.
Design: A randomised controlled trial.
Setting: A tertiary obstetric hospital in Melbourne, between July 2007 and May 2008.
Participants: 236 pregnant women recruited at ≤ 14 weeks’ gestation.
Intervention: Women allocated to the intervention group were given a personalised weight measurement card, advised of their optimal gestational weight gain (based on their body mass index at the time of recruitment and the United States Institute of Medicine guidelines), and instructed to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks’ gestation. The control group were weighed at recruitment, but were not given instructions about regular weight measurement. All participants were blinded to the purpose of the study.
Main outcome measure: Weight gain from recruitment to follow-up at 36 weeks’ gestation.
Results: In the study population, there was a trend to less weight gain in the intervention group. The women in the intervention group experienced a mean (SD) per-week weight gain of 0.44 (0.173) kg compared with those in the control group, who gained 0.46 (0.156) kg/week (mean difference, 0.02 kg/week; 95% CI, − 0.02 to 0.07 kg/week). The intervention significantly reduced gestational weight gain in the group of women who were overweight but not obese at recruitment: those in the intervention group (20 women) gained a mean (SD) of 0.42 (0.153) kg/week and the control group (18 women) gained 0.54 (0.123) kg/week (mean difference, 0.12 kg/week; 95% CI, 0.03 to 0.22 kg/week; P = 0.01).
Conclusion: Regular weight measurement in pregnancy was not found to be effective in reducing weight gain, except among women who were overweight but not obese before pregnancy.
Trial registration: Australian Clinical Trials Registry ACTRN12607000272493