Staphylococcus aureus sepsis, especially that caused by methicillin-resistant S. aureus (MRSA), is widely recognised by the public and the general medical community as a problem associated with health care. Less well recognised, but equally important, is invasive S. aureus infection arising in the community. The emergence of community strains of MRSA has added significant concern about community-onset infections.1 Although there is a wide variety of manifestations of serious invasive infections caused by S. aureus, in the great majority of these cases, the organism can be detected in blood cultures. Therefore, S. aureus bacteraemia is considered a very useful marker for these serious invasive infections,2 and in some cases, is the only initial manifestation of the infection.
Published reports of experience around the world show that mortality from infections associated with S. aureus bacteraemia can range from as low as 2.5% to as high as 40%.3-5 Mortality rates are known to vary significantly with patient age, clinical manifestation and comorbidities. Until recently, there have been no national data on the incidence and outcomes of S. aureus bacteraemia in either Australia or New Zealand. Small, largely retrospective, studies have been conducted, usually in single institutions. A recent prospective study of S. aureus bacteraemia conducted in 17 sites across Australia found a crude mortality rate of 11.2% when measured at discharge or 7 days, whichever came sooner.6 An earlier study that documented outcomes in six major centres in New Zealand found an all-cause mortality of 22.4% and an attributable mortality of 18.9%.7
The Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis (ANZCOSS) was established in 2007 to prospectively examine mortality from infections associated with S. aureus bacteraemia in a more structured way and to determine some of the risk factors for poor outcomes.8
Independent or hospital pathology laboratories in Australia and New Zealand were asked to join the cooperative on a voluntary basis. Invitations were extended to all members of the Australian Group on Antimicrobial Resistance,6 and by open invitation to selected hospital laboratories in New Zealand. The entry criterion for participating sites was a single blood culture that tested positive for S. aureus, associated with clinical manifestations in the patient consistent with staphylococcal infection. The date of study entry was the date of collection of the first positive culture in an episode of infection. A new episode in the same patient was recorded if the bacteraemia had cleared, but a further culture of blood taken more than 14 days after the initial positive culture was again positive.
Approval to conduct the prospective data collection was given by the research ethics committee associated with each participating laboratory. A web-based data entry system was constructed to enable real-time data collection into a common database. To ensure patient anonymity, but to allow follow-up of discrepant results with each participating site, a record identifier unique to the participating site was used. Data were collected on age, sex, ethnicity, date of admission (if admitted), date of discharge, relationship of the infection to a medical device and its type, the principal clinical manifestation of the infection, the principal agent used for definitive initial treatment (usually intravenous), and mortality at 7 and 30 days from date of entry. To avoid interpretive bias, no attempt was made to assign attributable mortality. Participating sites were asked to assign the susceptibility type of S. aureus to one of five types based on their locally generated antibiotic profile (Box 1). The place of onset of S. aureus infection was designated as the community (if the first positive blood culture in an episode of infection was collected before or within 48 hours of hospital admission) or hospital (if the first positive blood culture was collected more than 48 hours after admission).
Descriptive statistics were extracted in Microsoft Excel 2003 (Microsoft Corporation, Redmond, Wash, USA). Completed cases with mortality data available at 30 days were subjected to formal statistical analysis. Univariate analysis was conducted using χ2 and Fisher’s exact tests. Multivariable logistic regression analysis using a mixed effects model was undertaken using R version 2.8.1 (R Foundation for Statistical Computing; http://www.r-project.org/foundation/). The site of each laboratory was treated as a random effect, while all other predictors were treated as fixed effects. Independent variables included in the multivariable analysis were those identified as potentially significant on univariate analysis (P < 0.2).
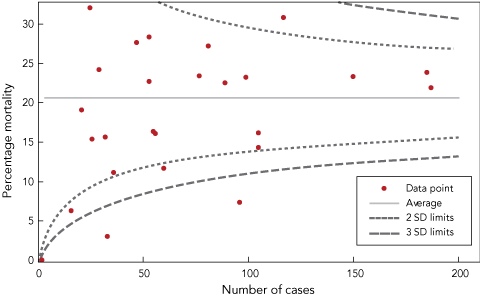
Percentage mortality was also compared for each participating site by using the funnel plot comparative assessment tool. This graphic quality assessment tool uses the average mortality rate for all cases combined, and constructs lines two SDs and three SDs either side of the average that are determined by the number of deaths and the total number of cases observed by each participating site.9
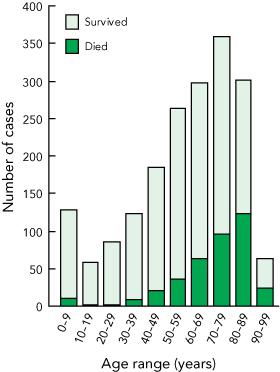
Univariate and multivariable analyses were performed on the 1865 cases for which 30-day all-cause mortality data were available. In the univariate analysis, demographic risk factors for increased mortality (Box 2) were age over 70 years and European ethnicity. As shown in Box 3, age-related mortality was a striking feature. Other significant factors on univariate analysis were some participating sites (Box 2); MRSA infection (other than non-multi-R MRSA); hospital-onset infection; infection that did not originate from a medical device; the clinical manifestations of sepsis syndrome, pneumonia/empyema and device infection with a metastatic focus; and treatment with a glycopeptide or other non-β-lactam (either for methicillin-susceptible S. aureus or MRSA infection) (Box 4).
Multivariable logistic regression using a mixed effects model showed that, of the significant risk factors identified in the univariate analysis, only age, place of onset of infection, a diagnosis of device infection with a secondary focus, principal clinical manifestation and treatment remained in the model (Box 5). Age was the strongest predictor of higher mortality, and both linear and quadratic terms in age were used to model the parabolic relationship between the variables. Pneumonia/empyema and sepsis syndrome were associated with higher mortality. Notably, we did not find evidence that infection with MRSA was an independent predictor of higher mortality. By contrast, glycopeptide treatment was predictive of higher mortality, with an odds ratio of 1.6 (95% CI, 1.1–2.7). Device-related infection and device type, when causing infection, were not independent predictors of reduced mortality in the multivariable analysis.
There was wide variation in mortality rates between participating sites. The results of the funnel plot comparison are shown in Box 6. Four participating sites had 30-day mortality rates below the 2 SD limit. Two sites had 30-day mortality rates below the 3 SD limit; for one of these, the finding could readily be attributed to age profile. One site had a 30-day mortality rate above the 2 SD limit. Investigation revealed a small spike of mortality attributable to late patient transfer to their site, while the subsequent mortality rate was close to the overall average.
This study provides the first reliable national estimate of percentage mortality from S. aureus bacteraemia for Australia, and provides indicative mortality for New Zealand. That so many institutions were willing to participate in the cooperative with no additional resources indicates the importance that those in the medical community attach to this problem. For Australia at least, we estimate that we have captured between a third and half of all cases over the period of data collection (about 1 year on average), based on previous estimates of the national incidence in Australia of around 6500 cases.5 The average 30-day mortality rate of around 20% is more than double that of bacterial diseases with a much higher public health profile, such as invasive meningococcal disease, and its incidence in the community is also substantially higher.10
In conducting this surveillance, we aimed to make data entry as simple and reliable as possible. To these ends, we chose web-based data entry because of its ready accessibility and consistency, and we kept the number of fields requiring data low. Further, we attempted to collect easily accessible data that required only a small additional effort above normal clinical activities, and to avoid, as far as possible, collecting data that required subjective interpretation. In particular, we did not ask participating sites to provide attributable mortality, which, in many respects, is a subjective judgement, particularly in cases with complex comorbidities. Some studies have estimated that attributable mortality is around 65% of crude mortality at 30 days.11 As an indication, the 7-day mortality rate of nearly 11% would almost all be attributable to staphylococcal sepsis.
More surprising is the fact that MRSA infection was not an independent risk factor for increased mortality in the multivariable analysis. A number of studies and meta-analyses have shown that MRSA is associated with poorer outcomes, including increased mortality.12 Our analysis suggests that this may be attributed to the antimicrobial agent used for treatment, almost always vancomycin. The likely reason that we obtained this result is that sufficient patients infected with methicillin-susceptible strains were treated with vancomycin instead of a β-lactam, and this allowed us to demonstrate a higher 30-day mortality with vancomycin than with flucloxacillin treatment. There has been increasing evidence of the inferior efficacy of vancomycin in treating staphylococcal infection,13 including recent compelling evidence of its inferiority to b-lactam treatment in treating methicillin-susceptible S. aureus infection.14 So, it may be better to consider MRSA as a major threat mostly because the drug of choice for serious invasive infection is less effective than β-lactams are against methicillin-susceptible strains. Unfortunately, new agents for treating serious MRSA infection, such as linezolid and daptomycin, have so far not shown predictable superiority over vancomycin in prospective randomised controlled trials.13 We have begun a retrospective review to determine why vancomycin does not perform as well as should be expected. Factors to consider include the pharmacokinetics and pharmacodynamics of the drug and the emergence of reduced susceptibility, especially of the heterogeneous type, in some strains of S. aureus.
A limitation of our study was that no data were collected on comorbidities. These have been shown to influence mortality significantly.11 Data such as these require more intensive efforts at collection, which we did not regard as conducive to recruitment or compliance with data collection. Moreover, the studies that have examined comorbidities have commonly used non-discriminatory measures, such as the Charlson Comorbidity Index, many components of which have no plausible link to poor outcomes for serious staphylococcal infection. Further studies are required to determine which aspects of comorbidities predict poor outcomes. It is possible that differences in comorbidities may have accounted in part for the differences in mortality rates we observed between participating sites.
1 Definitions of in-vitro susceptibility profiles for Staphylococcus aureus
2 Univariate analysis of demographic characteristics versus 30-day mortality for 1865 patients with Staphylococcus aureus bacteraemia
3 Number of cases of Staphylococcus aureus bacteraemia and patient survival, by age*
 | |||||||||||||||
|
* Data not shown for one patient aged 100 years, who survived. | |||||||||||||||
4 Univariate analysis of features of Staphylococcus aureus bacteraemia versus 30-day mortality in 1865 patients
PSSA = penicillin-susceptible S. aureus. MSSA = methicillin-susceptible S. aureus. Multi-R MRSA = multiresistant methicillin-resistant S. aureus. UK EMRSA-15-like = United Kingdom epidemic methicillin-resistant S. aureus type-15-like. Non-multi-R MRSA = non-multiresistant methicillin-resistant S. aureus. * By Pearson’s χ2 or Fisher’s exact test. † See Box 1 for definitions of susceptibility types. ‡ Includes osteomyelitis, septic arthritis and discitis. |
|||||||||||||||
5 Multivariable analysis of risk factors for 30-day all-cause mortality for patients with Staphylococcus aureus bacteraemia
Received 18 July 2008, accepted 11 June 2009
- John D Turnidge1
- Despina Kotsanas2
- Wendy Munckhof3
- Sally Roberts4
- Catherine M Bennett5
- Graeme R Nimmo6
- Geoffrey W Coombs7
- Ronan J Murray7
- Benjamin Howden8
- Paul D R Johnson8
- Kate Dowling1
- on behalf of the Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis
- 1 Women’s and Children’s Hospital, Adelaide, SA.
- 2 Monash Medical Centre, Melbourne, VIC.
- 3 Princess Alexandra Hospital, Brisbane; Ipswich Hospital, Ipswich; and University of Queensland, Brisbane, QLD.
- 4 Auckland City Hospital, and Starship Children’s Hospital, Auckland, NZ.
- 5 Melbourne School of Population Health, University of Melbourne, Melbourne, VIC.
- 6 Pathology Queensland, Royal Brisbane and Women’s Hospital, Brisbane, QLD.
- 7 PathWest Laboratory Medicine, Royal Perth Hospital, Perth, WA.
- 8 Austin Health, and University of Melbourne, Melbourne, VIC.
We are grateful to the Australian Society for Antimicrobials for funding the time of the data manager (Despina Kotsanas) and the set-up of the web-based data entry system. We are especially grateful to all those at the participating sites who gave their own time to enter the data and follow up discrepancies.
None identified.
- 1. Nimmo GR, Coombs GW, Pearson JC, et al. Methicillin-resistant Staphylococcus aureus in the Australian community: an evolving epidemic. Med J Aust 2006; 184: 384-388. <MJA full text>
- 2. Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother 2005; 56: 455-462.
- 3. Benfield T, Espersen F, Frimodt-Møller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 2007; 13: 257-263.
- 4. Frederiksen MS, Espersen F, Frimodt-Møller N, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J 2007; 26: 398-405.
- 5. Collignon P, Nimmo GR, Gottlieb T, Gosbell IB; Australian Group on Antimicrobial Resistance. Staphylococcus aureus bacteremia, Australia. Emerg Infect Dis 2005; 11: 554-561.
- 6. Turnidge JD, Nimmo GR, Pearson J, et al; Australian Group on Antimicrobial Resistance. Epidemiology and outcomes for Staphylococcus aureus bacteraemia in Australian hospitals, 2005–6: report from the Australian Group on Antimicrobial Resistance. Commun Dis Intell 2007; 31: 398-403.
- 7. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001; 31: 97-103.
- 8. Australian Society for Antimicrobials. Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis 2007. http://www.asa inc.net.au/anzcintro (accessed Aug 2009).
- 9. Spiegelhalter D. Funnel plots for institutional comparison. Qual Saf Health Care 2002; 11: 390-391.
- 10. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2004. Commun Dis Intell 2005; 29: 149-158.
- 11. Lesens O, Methlin C, Hansmann Y, et al. Role of comorbidity in mortality related to Staphylococcus aureus bacteraemia: a prospective study using the Charlson Weighted Index for comorbidity. Infect Control Hosp Epidemiol 2003; 24: 890-896.
- 12. Whitby M, McLaws M-L, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust 2001; 175: 264-267.
- 13. Cosgrove SE, Fowler VG Jr. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46 Suppl 5: S386-S393.
- 14. Kim S-H, Kim K-H, Kim H-B, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008; 52: 192-197.





Abstract
Objective: To document the types of, and mortality from, Staphylococcus aureus bacteraemia in Australia and New Zealand, and determine factors associated with mortality.
Design and setting: Prospective observational study in 27 independent or hospital pathology laboratories in Australia (24) and New Zealand (3), employing a web-based database to prospectively record demographic features, selected risk factors, principal antibiotic treatment and mortality data on all patients with positive blood cultures for S. aureus from June 2007 to May 2008.
Main outcome measure: 30-day all-cause mortality.
Results: 1994 episodes of S. aureus bacteraemia were identified, and complete 30-day follow-up data were available for 1865. Most episodes had their onset in the community (60.8%; 95% CI, 58.7%–63.0%). Methicillin-resistant S. aureus (MRSA) caused 450 episodes (24.1%; 95% CI, 22.2%–25.9%), and 123 of these (27.3%) had a susceptibility profile consistent with community-associated MRSA. All-cause mortality at 30 days was 20.6% (95% CI, 18.8%–22.5%). On univariate analysis, increased mortality was significantly associated with older age, European ethnicity, MRSA infection, infections not originating from a medical device, sepsis syndrome, pneumonia/empyema, and treatment with a glycopeptide or other non-β-lactam antibiotic. On multivariable analysis, independent predictors of mortality were age, sepsis syndrome, pneumonia/empyema, device-associated infection with a secondary focus, left-sided endocarditis, and treatment with a glycopeptide such as vancomycin, but not MRSA infection.
Conclusions: S. aureus bacteraemia is a common infection in both the community and hospitals in Australia and New Zealand, and is associated with appreciable mortality. Invasive MRSA infection may be more life-threatening, partly because of the inferior efficacy of the standard treatment, vancomycin. National web-based surveillance of S. aureus bacteraemia and its outcomes is not only important but also easily achievable.