Multidrug-resistant tuberculosis (MDR-TB) is a major threat to global TB control. It is defined as TB with resistance to at least isoniazid and rifampicin, two of the most effective first-line anti-TB drugs, and is initially caused by the improper use of these antibiotics during the treatment of patients with drug-susceptible TB.1 However, MDR-TB is also easily transmissible, so many patients now present with no prior history of TB treatment.2
According to the World Health Organization’s fourth global report on anti-TB drug resistance, there were an estimated 489 139 new cases of MDR-TB in 2006 (4.8% of all new cases of TB), with the greatest numbers of MDR-TB cases reported from China, India and Russia.2
Australia has a low incidence of MDR-TB.1 Between 1995 and 2006, the national incidence of MDR-TB ranged from four to 22 cases per year, which corresponds to 0.5%–2.4% of bacteriologically confirmed cases of TB.3 Most patients in Australia with MDR-TB were born overseas.3
In Victoria, most cases of TB occur due to the reactivation of latent infection in people who were infected in their country of birth.4 Hence, as rates of MDR-TB increase in countries from which Australia draws most of its migrants,5,6 so does the possibility that increased rates of MDR-TB will be seen in Victoria.
The management of patients with MDR-TB is intensive, expensive and prolonged, and associated with a greater likelihood of treatment failure than drug-susceptible TB.2 Therefore, even a small increase in the number of cases of MDR-TB presents numerous challenges for clinicians and public health officials.
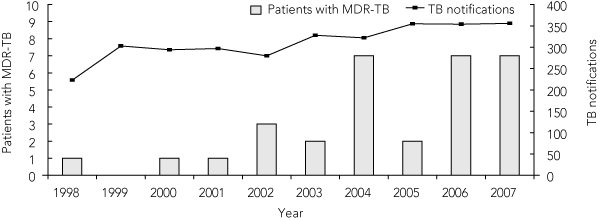
From 1998 to 2007, 31 patients in Victoria were diagnosed with MDR-TB. The annual number of new cases increased during the 10-year period, and the highest number of patients diagnosed with MDR-TB in any one year was seven (in 2004, 2006 and 2007) (Box 1). Cases of MDR-TB accounted for zero to 1.1% of all TB notifications in the first half of the decade, and 0.6%–2.2% of all TB notifications in the second half.
The median age of patients with MDR-TB was 27 years (interquartile range [IQR], 23–33 years), and 16 patients were male (Box 2). Twenty-nine patients were born overseas, of whom 27 were born in countries with a high risk of TB occurrence (Box 3).7 Patients from India, Vietnam and China accounted for almost two-thirds of overseas-born patients.
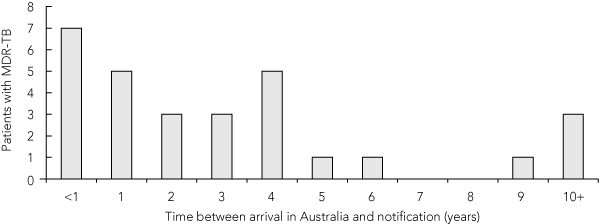
For overseas-born patients, the median time between arrival in Australia and notification of TB was 2 years (IQR, 1–4 years). Of the 29 overseas-born patients, 12 were notified to the Department of Health within 2 years of arrival in Australia (four of these patients were from India), and all but six within 5 years of arrival (Box 4).
Seventeen patients with MDR-TB had pulmonary disease only, 11 had extrapulmonary disease only, and three had both pulmonary and extrapulmonary disease (Box 5). In 12 patients with pulmonary disease (with or without extrapulmonary disease), sputum was smear-positive for acid-fast bacilli.
Our study revealed that there was a clear increase in the number of patients diagnosed with MDR-TB per year during the period 1998–2007. There was also a fivefold increase in the proportion of TB notifications that were accounted for by MDR-TB, which is consistent with national figures.3 New data, available since completion of our study, reveal that the increase in MDR-TB diagnoses appears to have been sustained in 2008 (six new cases of MDR-TB), although no new cases were diagnosed in the first 6 months of 2009 (TB Control Section, Victorian Department of Health, unpublished data).
If sustained, the increase in MDR-TB in Victoria will have significant implications for public health policy and planning. The management of MDR-TB involves the use of complex second- and third-line anti-TB drug regimens and, compared with drug-susceptible TB, prolonged periods of hospitalisation in negative-pressure rooms with specialist nursing care, multidisciplinary medical input and extensive use of laboratory services.8 The WHO estimates that the cost of treating a patient with MDR-TB is, on average, 100 times greater than for a patient with drug-susceptible disease.9 Investing in public health measures to prevent the transmission of MDR-TB, or to identify and treat secondary cases of both latent and active MDR-TB, can also have large financial implications.10 The finding that 12 of 20 patients with pulmonary disease in our study had smear-positive sputum, and were therefore likely to be highly infectious, highlights the importance of such measures.
TB control in Victoria and other jurisdictions of Australia is facilitated largely by migrant screening, epidemiological surveillance and bacteriological services.4 Although this approach is integral to effective TB control, our study raises questions about whether additional measures should be implemented. For example, given that the median delay between commencement of standard first-line therapy and second-line therapy was 61 days, one strategy that could be employed is the increased use of molecular tests for drug resistance. Rapid detection of drug resistance has a number of clinical and public health benefits, including earlier treatment of patients with the appropriate drug regimen, which reduces the overall period of treatment and the period of infectiousness.
As the median time between arrival in Australia and notification of TB was 2 years (indicating that some patients may have arrived with active TB), another strategy that might improve TB control is pre-departure screening for active TB, particularly in resource-limited settings. For example, the Australian Government Department of Immigration and Citizenship could develop guidelines for countries performing pre-migrant screening that outline minimum laboratory standards for TB culture testing for applicants with abnormal chest x-ray findings. Unfortunately, this strategy would still not detect most people entering Australia who have latent TB and normal chest x-ray findings when they obtain their visa (Box 6).
However, screening for latent TB will not necessarily provide a feasible solution either. Screening and treatment of migrants for latent TB as a method of TB control in low-incidence countries is a complex issue, and the evidence on clinical outcomes and cost-effectiveness of this approach is limited and contradictory.11-14 In addition, tests for latent TB, such as Mantoux tests or interferon γ release assays (eg, QuantiFERON-TB and enzyme-linked immunosorbent spot [ELISPOT] tests), do not distinguish latent TB that is fully sensitive to anti-TB drugs from MDR-TB. Isoniazid prophylaxis is also not likely to prevent reactivation of latent MDR-TB.
Ultimately, the elimination of TB in Australia and other low-incidence countries cannot be achieved without a coordinated global approach to TB control in high-incidence countries.15 The human impact and economic cost of MDR-TB means that continued vigilance and properly resourced community, state, national and international TB control programs remain essential.
1 Patients diagnosed with multidrug-resistant tuberculosis (MDR-TB) and all TB notifications by year, Victoria, 1998–2007
2 Patients diagnosed with multidrug-resistant tuberculosis (MDR-TB) by age and sex, Victoria, 1998–2007
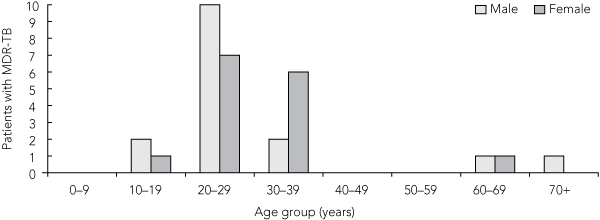
3 Patients diagnosed with multidrug-resistant tuberculosis (MDR-TB) and all TB notifications by country of birth, Victoria, 1998–2007
4 Overseas-born patients diagnosed with multidrug-resistant tuberculosis (MDR-TB) by time between arrival in Australia and notification of TB, Victoria, 1998–2007
5 Patients diagnosed with multidrug-resistant tuberculosis by site of disease, Victoria, 1998–2007
* Sputum was positive for acid-fast bacilli in 11 patients. † Sputum was positive in one patient. |
|||||||||||||||
Received 27 January 2009, accepted 30 April 2009
- Caroline J Lavender1
- Lynne K Brown2
- Paul D R Johnson3
- 1 Mycobacterium Reference Laboratory, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
- 2 TB Control Section, Victorian Department of Health, Melbourne, VIC.
- 3 Austin Health, Melbourne, VIC.
We thank James Fielding, Victorian Department of Health, for assistance with the ethics application, and Dr Janet Fyfe and Aina Sievers, Victorian Mycobacterium Reference Laboratory, for additional details on cases. We also thank staff in the School of Public Health, in particular Dr Priscilla Robinson, and students of the Master of Public Health program, at La Trobe University for helpful discussions.
None identified.
- 1. National Tuberculosis Advisory Committee. Multi-drug resistant tuberculosis: information paper (October 2007). Commun Dis Intell 2007; 31: 406-409.
- 2. World Health Organization. Anti-tuberculosis drug resistance in the world: fourth global report. Geneva: WHO, 2008. http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf (accessed Mar 2008).
- 3. Lumb R, Bastian I, Gilpin C, et al. Tuberculosis in Australia: bacteriologically confirmed cases and drug resistance, 2006: a report of the Australian Mycobacterium Reference Laboratory Network. Commun Dis Intell 2008; 32: 12-17.
- 4. Victorian Government Department of Human Services. Management, control and prevention of tuberculosis: guidelines for health care providers. Melbourne: DHS, 2002.
- 5. Australian Education International. International student enrolments in Australia — 1994 to 2006. Canberra: AEI, 2008. http://aei.dest.gov.au/AEI/MIP/Statistics/StudentEnrolmentAndVisaStatistics/2006/2006Annual_Stats.htm#1994 (accessed Sep 2008).
- 6. Australian Government Department of Immigration and Citizenship. Settler arrivals 1996–97 to 2006–07 — Australia, states and territories. Canberra: DIAC, 2007.
- 7. Australian Government Department of Immigration and Citizenship. Supplement to form 1163i. Complete country listings for the health assessment matrix. Canberra: Commonwealth of Australia, 2009. http://www.immi.gov.au/allforms/health-requirements/1163i-supplement.pdf (accessed Aug 2009).
- 8. White VL, Moore-Gillon J. Resource implications of patients with multidrug resistant tuberculosis. Thorax 2000; 55: 962-963.
- 9. Brown H. WHO identifies drug-resistant tuberculosis “hotspots”. Lancet 2004; 363: 951.
- 10. Moore-Gillon J. Multidrug-resistant tuberculosis: this is the cost. Ann N Y Acad Sci 2001; 953: 233-240.
- 11. Menzies D. Screening immigrants to Canada for tuberculosis: chest radiography or tuberculin skin testing? CMAJ 2003; 169: 1035-1036.
- 12. Khan K, Muennig P, Behta M, Zivin JG. Global drug-resistance patterns and the management of latent tuberculosis infection in immigrants to the United States. N Engl J Med 2002; 347: 1850-1859.
- 13. Schwartzman K, Menzies D. Tuberculosis screening of immigrants to low-prevalence countries. A cost-effectiveness analysis. Am J Respir Crit Care Med 2000; 161: 780-789.
- 14. Coker R, van Weezenbeek KL. Mandatory screening and treatment of immigrants for latent tuberculosis in the USA: just restraint? Lancet Infect Dis 2001; 1: 270-276.
- 15. Broekmans JF, Migliori GB, Rieder HL, et al. European framework for tuberculosis control and elimination in countries with a low incidence. Recommendations of the World Health Organization (WHO), International Union Against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J 2002; 19: 765-775.





Abstract
Objective: To describe demographic and clinical characteristics of patients residing in Victoria who were diagnosed with multidrug-resistant tuberculosis (MDR-TB) during the period 1 January 1998 to 31 December 2007.
Design, setting and patients: Retrospective review of Victorian Department of Health data on laboratory-confirmed cases of MDR-TB for the period 1998–2007.
Main outcome measures: Age, sex, country of birth, time between arrival in Australia and notification of TB, residency status, site of disease, and treatment period and outcome.
Results: From 1998 to 2007, 31 patients who resided in Victoria were diagnosed with MDR-TB. The median age of patients was 27 years, most patients were born overseas, and more than half were full-time students. The median time between arrival in Australia and notification of TB was 2 years, and 24 patients were notified to the Department within 5 years of arrival. Twenty patients had pulmonary disease; in 12 of these patients, sputum was smear-positive for acid-fast bacilli. The median treatment period for patients who completed treatment was 22 months.
Conclusions: The number of patients diagnosed with MDR-TB per year increased during the period 1998–2007. If sustained, this increase will have important implications for public health policy and planning.