Clinicians are responsible for ensuring that patients, or those legally responsible for patients’ care, volunteer consent in relation to intended procedures, having understood the nature and purpose of the procedure, material risks associated with the procedure, and alternative treatment options.1 Royal Australasian College of Surgeons guidelines appropriately highlight consent as a process, rather than a document.1 Nevertheless, the consent form remains a critical element in the process of obtaining patient consent for procedural interventions.2
The primary purpose of consent is not to minimise the risk of subsequent litigation, but to formalise the process by which patients (or those responsible for their care) are empowered to make appropriate decisions regarding treatment options. The goal of the consent process is to ensure that relevant information has been presented appropriately and understood. Written confirmation is not a mandated requirement of consent.1 In addition, the provision of written information may not necessarily improve subsequent patient recall.
In practice, several factors are highly relevant when imparting consent information: older people are over-represented as consumers of health care; limitations in sensory acuity or cognitive capacity may impede understanding; health care discussions may be stressful; opportunities for expansive discussion are frequently constrained by time, particularly in public hospital clinics; and patients may feel compelled to make significant treatment decisions in the absence of those who form their usual network of social support.
Audiotape analysis indicates that consent information provided by practitioners through verbal discussion is often deficient;3 studies show that the nature of consent information for specific procedures varies considerably between practitioners.4,5
For these reasons, we believe that a consent process is not acceptable unless verbal discussion is augmented by relevant written information. Specific documentation provides patients and others an opportunity to reconsider key points privately, or share information with “significant others”. It also ensures consistent and transparent provision of information.
Information provided on consent forms has not been studied widely. To our knowledge, no studies have assessed trends in the adequacy of consent documentation, despite the Royal Australasian College of Surgeons and indemnity insurers emphasising the importance of appropriate consent.
We reviewed the consent forms used in the Urology Unit at the Repatriation General Hospital, Adelaide. Adequacy of documentation related to the intended procedure, associated risks and treatment alternatives was assessed, and trends in the adequacy of consent documentation were analysed.
The Repatriation General Hospital provides urology services to the southern region of Adelaide — a catchment population of 330 000. During the study period, bookings for the elective surgery waiting list were made using a generic A3-sized form that comprised a patient consent form and a separate booking form for the waiting list manager. The procedure description was handwritten onto the form, and the patient received a carbon copy.
We undertook retrospective reviews of combined consent and booking forms for all patients on the Urology Unit waiting list on three occasions. Reviews were undertaken during 2005, 2007 and 2008, with a minimum of 12 months between reviews. The criteria assessed were: procedure description, including use of technical terminology, acronyms and plain language; completeness with respect to the description of the intended procedure; inclusion of information regarding the purpose of the procedure, risks associated with the procedure, and alternative treatments. The procedure description on the patient consent form was also compared with that provided to the waiting list manager on the adjacent booking form.
Descriptions were regarded as technical if the terminology employed was not in general community use or not contained in a standard dictionary,6 and plain language descriptions were defined as those for which technical descriptors of procedures were in common use (eg, circumcision, vasectomy). Consent forms were regarded as incomplete if significant information regarding the intended procedure had been omitted; this was determined on the basis of the accompanying diagnosis, or the booking information provided to the waiting list manager. Forms were still considered complete if they used acronyms and technical terminology, as long as they included a description of all elements of the procedure.
The study was approved by the Repatriation General Hospital Research and Ethics Committee.
Combined consent and booking forms were reviewed for 1854 patients, of which 574 forms were excluded as the consent form had not been filled out at all. Forms for 17 patients who were booked to undergo radical prostatectomy or radical cystectomy were also excluded, as these patients receive additional information from educational booklets, allied health providers and nursing staff.
The remaining 1280 forms were analysed, comprising 352 for 2005, 491 for 2007 and 437 for 2008. No trend in the studied criteria of adequacy of documentation was observed during the study period (Box 1).
With few exceptions, the carbon copy of the combined consent and booking form was the only written information regarding the intended procedure, risks and benefits that was provided to the patient.
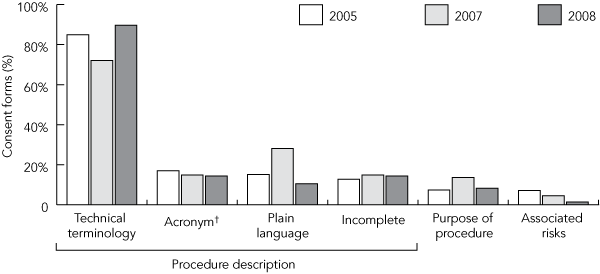
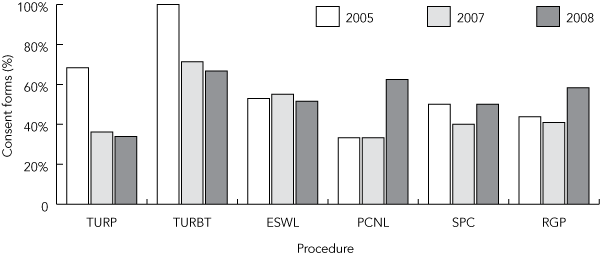
Description of procedure. Technical descriptions of the intended procedure, alone or combined with acronyms, were used in 81.6% of consent forms (Box 1). In 15.3% of consent forms, a significant component was described using an acronym, without provision of any further explanation (Box 1). In 6.6% of consent forms, the description consisted entirely of abbreviations, acronyms or technical terminology, with no plain language. The frequent use of acronyms within the surgical specialty was particularly common in consent descriptions for six specific procedures (Box 2). Collectively, these six procedures accounted for 28.0% of all procedures. Plain language descriptions (Box 3) were provided in 18.5% of consent forms (Box 1). No consent form provided a plain language description of the proposed procedure together with information regarding associated risks and alternative treatments.
Completeness. Procedure descriptions were complete in 85.9% of consent forms (Box 1). The nature of omitted information varied. In 28.2% of forms, the description did not mention the side of the procedure. In 71.8% of consent forms, the location of the intended procedure was not specified. In 26.5% of consent forms, activity related to an unspecified prosthesis was mentioned (eg, “cystoscopy and insertion of stent”).
Purpose of procedure. The purpose of the procedure was included in 10.1% of consent forms (Box 1). In this analysis, descriptions such as “bladder telescope inspection” and “circumcision” were not automatically included, as these procedures may be performed for a variety of reasons.
Associated risks. Information relevant to risks associated with the intended procedure was present in 4.1% of consent forms (Box 1). One of these conveyed information about risks using a structured sentence, and the others used word lists. Four consent forms included an estimate of the likelihood that an adverse event would occur.
Alternative treatments. No consent form included information regarding the availability of alternative treatments, or the implication of declining intervention.
Differential description. The procedure description provided on the consent form differed to that provided to the waiting list manager on the booking form in 55.7% of forms (41.8% for 2005, 65.2% for 2007, 29.1% for 2008). In 56.8% of these forms (54.4% in 2005, 33.4% in 2007, 61.9% in 2008) the change entailed technical expansion of an acronym or abbreviation (eg, ESWL on booking form became extracorporeal shockwave lithotripsy on consent form) or different wording on the consent form that persisted in the use of technical language. Plain language descriptions on consent forms were used in 29.2% of forms within this category (32.7% in 2005, 23.0% in 2007, 22.7% in 2008). Differences in the remaining forms were due to omission of specific anaesthetic or technical information on consent forms, or minor grammatical variations.
In this study, the description of intended procedures was most often provided in a format that could not be interpreted without specialist medical knowledge. As a result, most patients did not receive any information resource that would facilitate later reflection or discussion. No consistent trend towards improved standards of documentation was observed over the 3-year period.
Observed practice fell short of the standards that we believe would be expected by indemnity insurers and the community. We believe the majority of consent documents that we reviewed would be unlawful in most non-medical contractual contexts. It is disturbing that about one in 15 patients did not receive a procedure description on the consent form that could be interpreted even with reference to a standard dictionary.
The use of technical jargon or acronyms on patient consent forms is unacceptable. This underscores a view of consent as a documentary hurdle, rather than an educational opportunity. In about half the forms that we reviewed, identical technical descriptions of the intended procedure were provided to the patient and waiting list manager. This suggests that the differing capacity of each to interpret abbreviations and jargon was not considered. Even when attempts were made to expand the description for the benefit of the patient, most documents persisted with use of technical terminology or acronyms.
A recent United States study of two urological procedures also showed significant deficiencies in the provision of information related to consent.7 Overall, however, information disclosure was far more comprehensive than in our review. For example, 51% of patients received at least some written information regarding treatment alternatives.7 To our knowledge, no study has reviewed consent documentation across a broad spectrum of procedures.
Ultimately, the provision of appropriate information is an issue of social responsibility. In settings other than health care, consumers of products or services are typically cognisant of documentation in terms of warranties, explanations or descriptions. In contrast, critical appraisal of the consent process does not usually occur in acute care settings. Despite the efforts of clinicians, the consent process may be perceived as threatening. We do not believe that the community would endorse a system that allows patients to undergo complex and potentially morbid treatment when the only information provided for private reflection is a non-English acronym.
In response to our findings, structured consent forms and patient information sheets containing identical information have been embedded within procedure-specific elective surgery booking forms for the Urology Unit at the Repatriation General Hospital. This linkage ensures that information relevant to consent is necessarily generated with every patient booking. The use of preprinted forms is also more efficient for practitioners than handwritten descriptions. This does not diminish the duty of clinicians to ensure that specific risks relevant to individual patients have been addressed.
Our study did not measure or account for information provided verbally at the time consent was obtained. Neither did it measure the impact of more detailed documentation on patient recall. Rather, we have identified that most patients consented to undergo procedural intervention without the provision of any useful written information resource. Many tools are available to facilitate the provision of information relevant to consent. These include information sheets endorsed by the Royal Australasian College of Surgeons, and more specific information provided by individual clinicians. Previous studies have emphasised the importance of addressing both readability and content.8,9
We acknowledge that a detailed document does not equate to an adequate consent process.10 However, we believe the extent of information provided should be governed by mandatory practice standards. We propose that the minimum requirements for valid consent documentation in elective procedures should include:
a plain language description of the intended procedure;
an explanation of the purpose and intended benefit;
information regarding material risks; and
information regarding established treatment alternatives.
The present findings reflect the spectrum of consent documentation in one hospital unit. However, we believe that the practices we have observed are endemic in Australian public hospitals. In the past decade, there has been a burgeoning interest in redesigning the process by which care is provided in Australian hospitals. We would welcome broader review of the extent to which consent principles are translated into practice, and we propose that the community should consider:
Is an acronym or technical terminology appropriate for documenting consent?
If not, can minimum practice standards for documentation be defined?
Should any emerging recommendations be mandated?
The principles and practice of informed consent are critical issues for the medical profession and the community. Verbal information provided by clinicians is often deficient, and there is no uniformity in regard to the nature or extent of information that is provided. At present, the provision and documentation of information is discretionary and unregulated. We argue that the best interests of the community, and ultimately the medical profession, would be served by implementation of mandatory practice standards for documentation of procedural consent.
1 Proportions of consent forms for a urology unit waiting list according to language used in procedure description, completeness of procedure description, and inclusion of information on procedure purpose and associated risks*
 |
|
* Data represent three reviews, undertaken during 2005, 2007 and 2008, with a minimum of 12 months between reviews. † Data represent consent forms on which a significant component was described using an acronym, without provision of any further explanation. |
2 Proportions of consent forms for a urology unit waiting list that used only acronyms to describe common urological procedures*
 |
|
* Data represent three reviews, undertaken during 2005, 2007 and 2008, with a minimum of 12 months between reviews. TURP = transurethral resection of the prostate. TURBT = transurethral resection of bladder tumour. ESWL = extracorporeal shockwave lithotripsy. PCNL = percutaneous nephrolithotomy. SPC = suprapubic catheter (insertion). RGP = retrograde pyelogram. |
3 Examples of terminology used for procedure descriptions on consent forms for a urology unit waiting list
Technical descriptions
Extracorporeal shockwave lithotripsy
Ureteropyeloscopy
Diverticulectomy
Acronyms
TURP
PCNL
RGP
Plain language
Circumcision
Telescope inspection of bladder
Removal of kidney for cancer
TURP = transurethral resection of the prostate.
PCNL = percutaneous nephrolithotomy.
RGP = retrograde pyelogram.
Received 3 May 2009, accepted 30 June 2009
- Mark T Siddins1
- Elizabeth M Klinken2,3
- Lee R Vocale2,4
- 1 Urology Unit, Repatriation General Hospital, Adelaide, SA.
- 2 Flinders University School of Medicine, Adelaide, SA.
- 3 Sir Charles Gairdner Hospital, Perth, WA.
- 4 Austin Hospital, Melbourne, VIC.
None identified.
- 1. Royal Australasian College of Surgeons. Informed Consent Policy. Melbourne: RACS, 2008.
- 2. Royal Australasian College of Surgeons. Royal Australasian College of Surgeons implementation guidelines for ensuring correct patient, correct side and correct site surgery. Melbourne: RACS, 2008.
- 3. Braddock CH, Edwards KA, Hansberg NM, et al. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999; 282: 2313-2320.
- 4. McManus PL, Wheatley KE. Consent and complications: risk disclosure varies widely between individual surgeons. Ann R Coll Surg Engl 2003; 85: 79-82.
- 5. Seow CH, Leber JM, Ee HC, Yusoff IF. Survey of consent practices for inpatient colonoscopy and endoscopic retrograde cholangiopancreatography at a tertiary referral centre. J Gastroenterol Hepatol 2006; 21: 1340-1345.
- 6. Ludowyk F, Moore B, editors. The Australian modern Oxford dictionary. 2nd ed. Melbourne: Oxford University Press, 2003.
- 7. Issa MM, Setzer E, Charaf C, et al. Informed versus uninformed consent for prostate surgery: the value of electronic consents. J Urol 2006; 176: 694-699.
- 8. Bottrell MM, Alpert H, Fischbach RL, Emanuel LL. Hospital informed consent for procedure forms: facilitating quality patient–physician interaction. Arch Surg 2000; 135: 26-33.
- 9. Hopper KD, TenHave TR, Tully DA, Hall TE. The readability of currently used surgical/procedure consent forms in the United States. Surgery 1998; 123: 496-503.
- 10. Stanley BM, Walters DJ, Maddern GJ. Informed consent: how much information is enough? Aust N Z J Surg 1998; 68: 788-791.





Abstract
Objective: To determine the adequacy of consent documentation related to descriptions of intended procedures, associated risks and treatment alternatives, and to analyse trends in the adequacy of consent documentation in a specialty surgical unit.
Design, patients and setting: Retrospective reviews of consent forms for all patients on the Urology Unit waiting list of the Repatriation General Hospital, Adelaide on three occasions. Reviews were undertaken during 2005, 2007 and 2008, with a minimum of 12 months between reviews.
Results: 1280 consent documents were evaluated. No trend in the studied criteria of adequacy of documentation was observed during the study period. Overall, 18.5% of consent forms described procedures using plain language. In 15.3% of consent forms, a significant component of the procedure was described using only an acronym, without further explanation. In 6.6% of consent forms, procedure descriptions contained only acronyms, abbreviations or technical terminology, with no plain language word. The purpose of the operation was conveyed in 10.1% of consent forms. Relevant risks were provided in 4.1%. Any indication of the magnitude of procedural risks was provided in only four of 1280 forms. No consent form provided information about alternative treatments.
Conclusions: We believe these findings are broadly representative of current hospital practice and that the community should consider whether an acronym or technical terminology is appropriate for documenting consent. If not, can minimum practice standards be defined, and should any emerging recommendations be mandated?