Stent placement is one of the most common procedures performed in urological practice, as a stand-alone procedure to relieve renal obstruction, or as an adjunct to an increasing array of complex endourological procedures.1 Although urological stents are well tolerated by most patients, stent-related complications are frequent and a common cause of patients re-presenting. The most common side effects are irritative voiding symptoms, suprapubic discomfort and haematuria, usually related to irritation from the bladder coil, or flank pain related to urinary reflux on micturition. Long-term stents, particularly those placed for the management of ureteric strictures or compression not amenable to reconstruction, are prone to encrustation, blocking and subsequent infection.2
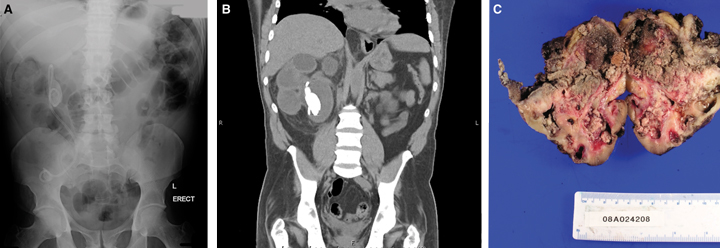
The prospect of a “missed” stent is a constant source of anxiety to the practising urologist. Stents that have been inadvertently left in situ for many months act as niduses for stone formation, particularly at the proximal and distal coils, which may prevent easy stent removal. Encrustation increases stent fragility and may lead to its fragmentation, even during careful attempts at removal. Multiple procedures may be required to remove the stent completely. Encrustation may also impair urine drainage, resulting in unrecognised renal obstruction and silent kidney damage, necessitating nephrectomy. As in our patient, long-term obstruction may cause persistent infection and recurrent episodes of clinical pyelonephritis and subsequent pyonephrosis. From a biological viewpoint, it is not surprising that the combination of stent irritation and infection would lead eventually to malignant transformation,3 although to our knowledge this is the first reported case of ureteric carcinoma associated with a retained ureteric stent.
Many strategies have been proposed to prevent the occurrence of “missed” stents. In current Australian practice, clinicians rely on a paper-based system that requires manual entry and interrogation to identify overdue stents, and in general this system operates efficiently. However, in a large tertiary hospital where health care is often delivered by a number of different visiting medical officers or junior medical staff, difficulties with a paper-based system may arise. In our experience, the main difficulty is capturing data in unusual circumstances; for example, a stent placed either out-of-hours or at the request of other services in non-urological theatres may not be entered into the system. Recently, a computerised system has been described that addresses this problem.4 However, it is yet to be seen if this can be successfully introduced into other institutions.
Lessons from practice
Missed ureteric stents are a frequent source of morbidity, repeated procedures and occasional mortality.
No stent should be placed without an appropriate removal plan.
Retained stents can cause recurrent urinary tract infections, which are typically difficult to clear; occasionally, they cause flank pain, fevers and night sweats secondary to suppurative infection.
Patients with urinary tract infection and a history of urological intervention, particularly for stone disease, need a plain x-ray of the abdomen.
Identification on x-ray of a stent that has remained in situ for longer than 6 months (unless this was planned) should prompt urgent referral to an appropriate urology service for removal.
- 1. Russell JM, Clarke SJ, Boyce WH. The use of the ureteric stent. Aust N Z J Surg 1978; 48: 327-330.
- 2. Richter S, Ringel A, Shalev M, et al. The indwelling ureteric stent: a ‘friendly’ procedure with unfriendly high morbidity. BJU Int 2000; 85: 408-411.
- 3. Costerton JW, Chenq KJ, Geesey GG, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol 1987; 41: 435-464.
- 4. Lynch MF, Ghani KR, Frost I, et al. Preventing the forgotten ureteral stent: implementation of a web-based stent registry with automatic recall application. Urology 2007; 70: 423-426.





None identified.