A 6-week old infant who had been conceived through in-vitro fertilisation (IVF) presented with a skin lesion and enlarged lymph nodes, and developed severe respiratory distress. Mycobacterium tuberculosis was identified; his mother was the only potential source identified. To our knowledge, this is the first case of congenital tuberculosis after IVF reported in Australia and the second worldwide. It highlights the importance of adequate screening during investigation of infertility and the difficulties in diagnosing congenital tuberculosis.
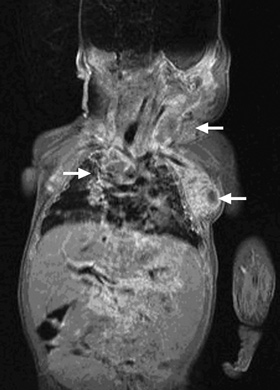
At 6 weeks of age, while still in hospital, the infant developed a 1 cm brown–pink macular lesion on the left side of the neck, and was noted to have an enlarged (1.5 cm) left axillary lymph node. Empirical antistaphylococcal therapy was introduced without any apparent effect. Biopsy of the lymph node was planned, but before this could be performed, the lymph node became acutely more enlarged, and the infant’s condition deteriorated rapidly. He developed severe respiratory distress and required re-intubation. A large mediastinal lymph node mass was noted on chest x-ray and magnetic resonance imaging (Box 1), and bronchoscopy showed extrinsic compression of the trachea and bronchi at the level of the carina.
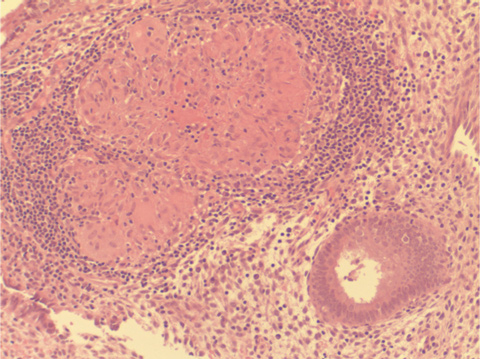
The child’s mother had no significant medical history apart from infertility. Infertility investigations had included ultrasound, hysteroscopy and diagnostic laparoscopy. A uterine curettage (as part of the work-up for infertility) 5 years before the pregnancy had shown “granulomas” in the endometrium (Box 2), but stains for acid-fast bacilli were negative. Culture for M. tuberculosis had not been performed at the time. Following the diagnosis of tuberculosis (TB) in her child, an interferon-gamma release assay (IGRA [QuantiFERON-TB Gold, Cellestis, Melbourne, Vic]) was performed, and gave a positive result. Chest x-ray revealed old fibrotic changes in the right upper lung, but no evidence of active tuberculous disease. She was prescribed antituberculous medication.
This is the first case of congenital TB after IVF reported in Australia, and to our knowledge only the second case reported in the literature.1 This case suggests two valuable lessons: the importance of considering congenital TB in babies who develop a suggestive clinical illness in the first weeks of life, and the need for accurate assessment for and exclusion of TB in women proceeding to IVF.
Congenital TB is a rare condition, with around 350 cases reported in the literature.2-10 The fetus can be infected by direct spread through the umbilical cord, by aspiration or swallowing of infected amniotic fluid, or by direct contact with maternal genital lesions during delivery.11 TB can be difficult to recognise in infants as symptoms may be non-specific and easily mistaken for more common neonatal illnesses, such as bacterial sepsis or congenital viral infections.12 Symptoms usually present 2–3 weeks after birth, and the disease can quickly progress to dissemination and death if not promptly treated.
In 1994, criteria for diagnosis of congenital TB were revised.3 They comprise documentation of tuberculous lesions in the infant and one or more of the following: lesions in the first weeks of life; a primary hepatic complex or caseating granuloma; documented tuberculous infection of the endometrium or placenta; or exclusion of the possibility of postnatal transmission by investigation of close contacts. Our patient met the criteria for congenital TB: he had tuberculous lesions in early life; his mother had documented granulomas of the endometrium; and extensive contact tracing found no evidence of transmission to the child from other close contacts. Confirmation of endometrial TB in the mother, by repeat endometrial biopsy with PCR and culture for TB, would have been ideal, but would not have altered management in either the mother or the neonate. Thus, it was elected to treat the mother on the basis of the neonate’s culture results, rather than subject her to another procedure.
Globally, there has been a slow decline in the incidence of TB; but this is more than offset by population growth, with the number of new cases worldwide increasing between 2005 and 2006 from 9.1 to 9.2 million (an increase of 0.6%). Increases occurred in the African, Eastern Mediterranean, European and South-East Asian regions.13 In Australia, the total number of TB cases reported in 2006 was 1201 (5.8 cases per 100 000 population). The incidence varies dramatically between populations in Australia, with 0.9 cases per 100 000 population in the non-Indigenous population, climbing to 20.1 per 100 000 population in those born overseas.14 Within the group born overseas, incidence ranges from 2.0 per 100 000 population for those born in the United Kingdom to 405 per 100 000 population for those born in Somalia.14
Genital TB is a major cause of infertility in women belonging to high-risk groups, causing up to 17% of cases of infertility.15 Its incidence is increasing in Western continents. Twenty-nine cases of genitourinary TB were reported in Australia in 2006 (2.5% of all reported cases of TB).14 In Australia, it is estimated that one in every 60 babies is conceived through IVF .16 As IVF is a useful treatment for infertility caused by TB, it can be expected that in the future more women with TB as a cause of their infertility will present for IVF. It is therefore imperative that women from high-risk groups undergo evaluation for and exclusion of TB. Investigations should include an IGRA or tuberculin skin test. If either gives a positive result, expert opinion should be sought to determine the need for more invasive investigations, such as endometrial biopsy. If an endometrial biopsy reveals granuloma, it is imperative that the specimen be sent for PCR and culture for TB, even in the absence of acid-fast bacilli. Consultation with an infectious diseases physician or clinical microbiologist is then warranted.
- 1. Doudier B, Mosnier E, Rovery C, et al. Congenital tuberculosis after in-vitro fertilization. Pediatr Infect Dis J 2008; 27: 277-278.
- 2. Snider D, Bloch A. Congenital tuberculosis. Tubercle 1984; 65: 81-82.
- 3. Cantwell M, Shehab Z, Costello A, et al. Congenital tuberculosis. N Engl J Med 1994; 330: 1051-1054.
- 4. Adhikari M, Pillay T, Pillay DG. Tuberculosis in the newborn: an emerging disease. Pediatr Infect Dis J 1997; 16: 1108-1112.
- 5. Lee LH, LeVea CM, Graman PS. Congenital tuberculosis in a neonatal intensive care unit: case report, epidemiological investigation, and management of exposures. Clin Infect Dis 1998; 27: 474-477.
- 6. Laartz BW, Narvarte HJ, Holt D, et al. Congenital tuberculosis and management of exposures in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2002; 23: 573-579.
- 7. Pillay T, Adhikari M. Congenital tuberculosis in a neonatal intensive care. Clin Infect Dis 1999; 29: 467-468.
- 8. Hatzistamatiou Z, Kaleyias J, Ikonomidou U, et al. Congenital tuberculous lymphadenitis in a preterm infant in Greece. Acta Paediatr 2003; 92: 392-394.
- 9. Crockett M, King SM, Kitai I, et al. Nosocomial transmission of congenital tuberculosis in a neonatal intensive care unit. Clin Infect Dis 2004; 39: 1719-1723.
- 10. Mouchet F, Hansen V, Van Herreweghe I, et al. Tuberculosis in healthcare workers caring for a congenitally infected infant. Infect Control Hosp Epidemiol 2004; 25: 1062-1066.
- 11. Starke J. Tuberculosis: an old disease but a new threat to the mother, fetus and neonate. Clin Perinatol 1997; 24: 107-127.
- 12. Mazade M, Evans E, Starke J, Correa A. Congenital tuberculosis presenting as a sepsis syndrome: case report and review of the literature. Pediatr Infect Dis J 2001; 20: 439-442.
- 13. World Health Organization. Global tuberculosis control 2008. http://www.who.int/tb/publications/global_report/2008/en/index.html (accessed Dec 2008).
- 14. Roche PW, Krause V, Konstantinos A, et al. Tuberculosis notifications in Australia, 2006. Commun Dis Intell 2008; 32: 1-11.
- 15. Tripathy S, Tripathy S. Infertility and pregnancy outcome in female genital tuberculosis. Int J Gynaecol Obstet 2002; 76: 159-163.
- 16. Van Voorhis BJ. Clinical practice. In vitro fertilization. N Engl J Med 2007; 356: 379-386.





None identified.