Coeliac disease (CD) is an autoimmune disease, triggered by the ingestion of gluten in genetically susceptible individuals, and causing an inflammatory reaction and damage to the small intestine. The diagnostic “gold standard” is the normalisation of the small intestinal mucosa when gluten is eliminated from the diet. Population studies have shown that CD is relatively common in white populations, with about 0.5%–1% estimated to have undetected CD.1-3
In 2001, our group published the results of anti-endomysial antibody (anti-EMA) testing of 3011 adults in the Busselton (Western Australia) population, finding a prevalence of CD of about 0.4% (1 : 250).4 The Busselton Health Study comprises a series of cross-sectional general population surveys conducted every 3 years from 1966 to 1981, with a follow-up study of all available previous participants in 1994–1995. At this follow-up, serum samples suitable for serological and HLA testing were taken for long-term storage. These samples were used in our 2001 study.4
Studies of CD prevalence with anti-EMA testing have shown a wide variation in the sensitivity (74%–100%) and specificity (64%–100%) of this test, which may have arisen from differences in the laboratory techniques employed.5-11 These variations suggest that anti-EMA testing may not be suitable as a screening tool. Testing for human anti-tissue transglutaminase (anti-tTG) antibodies appears to give more consistent results and is likely to be the best test for CD; initial reports have shown a sensitivity of 95%–98% and a specificity of 94%.12,13 An even higher sensitivity (99.5%) and specificity (99.6%) have been achieved using recombinant human anti-tTG in a radioligand assay,14 confirming the reliability of anti-tTG testing.15
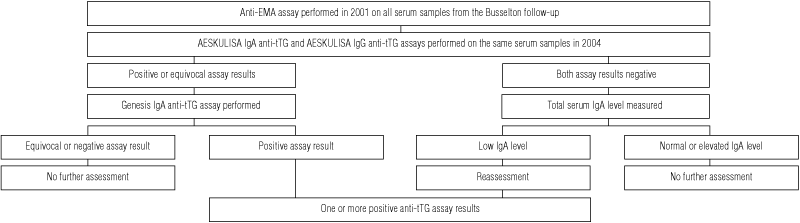
In December 2004, we performed AESKULISA IgA and IgG anti-tTG assays (AESKU.DIAGNOSTICS, Wendelsheim, Germany) on the 3011 stored serum samples of subjects attending the 1994–1995 Busselton Health Study follow-up (the same samples tested for anti-EMA in our 2001 study).4
Serum samples giving at least one positive result, or equivocal results, of the AESKULISA IgA anti-tTG assay (cut-off, > 10 U/mL) and the AESKULISA IgG anti-tTG assay (cut-off, > 15 U/mL) were tested with the Genesis IgA anti-tTG assay (cut-off > 7 U/mL; Genesis Diagnostics Ltd, Cambridge, UK). In samples with negative results of the anti-tTG assays, serum IgA levels were measured. The procedure is summarised in Box 1.
Available subjects were reviewed by a gastroenterologist between December 2004 and July 2005, and a symptom questionnaire was completed. Blood was taken for repeat AESKULISA IgA/IgG anti-tTG and Genesis IgA anti-tTG testing; HLA-DQ2 and HLA-DQ8 haplotyping; full blood examination; measurement of serum ferritin, vitamin B12, vitamin D and blood sugar levels; and liver function tests.
In unavailable subjects, HLA-DQ2 and HLA-DQ8 haplotyping was performed on their stored sera.
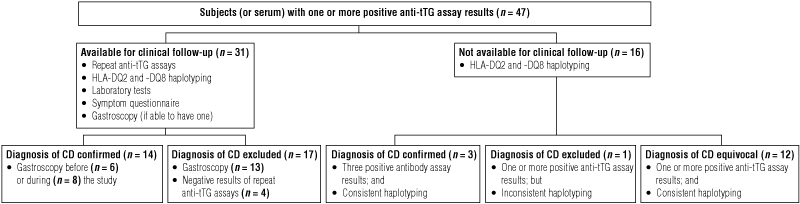
Forty-seven serum samples gave positive results for at least one anti-tTG assay, a prevalence of 1.56%. The serological and duodenal biopsy results of the 47 subjects with at least one positive result of anti-tTG assays are summarised in Box 2.
We found that the prevalence of anti-tTG antibodies in a predominantly Anglo-Celtic Australian population was 1.56%. This is about three times higher than the prevalence of anti-EMA that we reported in the same population in 2001.4 To our knowledge, this is the largest study published to date of the prevalence of anti-tTG antibodies and CD in a general healthy population. About two-thirds of the subjects with positive results of at least one anti-tTG assay were reviewed clinically, and just over two-thirds of the reviewed group underwent upper gastrointestinal gastroscopy, and distal duodenal biopsy.
This cohort of subjects has been followed up for up to 40 years as part of the Busselton Health Study. In 27 subjects, positive anti-tTG assay results were obtained in two serum samples taken with a gap of 12 years between them. Despite this long period of stable seropositivity, we were able to definitely refute the diagnosis of CD in 17 of these subjects by gastroscopy or negative results of repeat anti-tTG assays. Latent or potential CD is an entity that is sometimes used to describe patients with few or no symptoms, abnormal results of serological tests for CD and normal histological findings.17 Although there is debate and controversy about how to manage these patients,18 we have shown in our study that a significant proportion of them do not go on to develop active CD.
- Marcus W Chin1
- Dominic F Mallon2
- Digby J Cullen3
- John K Olynyk4
- Lindsay C Mollison4
- Callum B Pearce4
- 1 Department of Hepatology, Sir Charles Gairdner Hospital, Perth, WA.
- 2 PathWest, Fremantle and Princess Margaret Hospitals, Perth, WA.
- 3 Fremantle Hospital, Perth, WA.
- 4 University of Western Australia, Perth, WA.
We thank the Busselton Health Study Committee and the people of Busselton for their participation and assistance in this study.
None identified.
- 1. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003; 163: 286-292.
- 2. Fasano A. European and North American populations should be screened for coeliac disease. Gut 2003; 52: 168-169.
- 3. Maki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med 2003; 348: 2517-2524.
- 4. Hovell CJ, Collett JA, Vautier G, et al. High prevalence of coeliac disease in a population-based study from Western Australia: a case for screening? Med J Aust 2001; 175: 247-250.
- 5. Ladinser B, Rossipal E, Pittschieler K. Endomysium antibodies in coeliac disease: an improved method. Gut 1994; 35: 776-778.
- 6. Corrao G, Corazza GR, Andreani ML, et al. Serological screening of coeliac disease: choosing the optimal procedure according to various prevalence values. Gut 1994; 35: 771-775.
- 7. Cataldo F, Ventura A, Lazzari R, et al. Antiendomysium antibodies and coeliac disease: solved and unsolved questions. An Italian multicentre study. Acta Paediatr 1995; 84: 1125-1131.
- 8. Carroccio A, Cavataio F, Iacono G, et al. IgA antiendomysial antibodies on the umbilical cord in diagnosing celiac disease. Sensitivity, specificity, and comparative evaluation with the traditional kit. Scand J Gastroenterol 1996; 31: 759-763.
- 9. Parnell ND, Ciclitira PJ. Coeliac disease and its management. Aliment Pharmacol Ther 1999; 13: 1-13.
- 10. James MW, Scott BB. Endomysial antibody in the diagnosis and management of coeliac disease. Postgrad Med J 2000; 76: 466-468.
- 11. Bowron A, Moorghen M, Morgan JE, et al. Cost-effective strategy for the serological investigation of coeliac disease. Ann Clin Biochem 2000; 37 (Pt 4): 467-470.
- 12. Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998; 115: 1322-1328.
- 13. Dieterich W, Laag E, Schopper H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology 1998; 115: 1317-1321.
- 14. Seissler J, Schott M, Boms S, et al. Autoantibodies to human tissue transglutaminase identify silent coeliac disease in type I diabetes. Diabetologia 1999; 42: 1440-1441.
- 15. King AL, Yiannakou JY, Brett PM, et al. A genome-wide family-based linkage study of coeliac disease. Ann Hum Genet 2000; 64 (Pt 6): 479-490.
- 16. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”). Gastroenterology 1992; 102: 330-354.
- 17. Troncone R, Greco L, Mayer M, et al. Latent and potential coeliac disease. Acta Paediatr Suppl 1996; 412: 10-14.
- 18. Murdock AM, Johnston SD. Diagnostic criteria for coeliac disease: time for change? Eur J Gastoenterol Hepatol 2005; 17: 41-43.





Abstract
Objectives: To determine (i) the prevalence of positive results of anti-tissue transglutaminase (anti-tTG) antibody assays and coeliac disease (CD) in a rural Australian community; and (ii) whether confirmatory testing of a positive assay result with an alternative anti-tTG assay improved the positive predictive value of the test in population screening for CD.
Design: Retrospective analysis in December 2004 of stored serum samples taken in 1994–1995 from 3011 subjects in the Busselton Health Study follow-up. Assays for IgA and IgG anti-tTG antibodies were performed, and positive or equivocal samples were retested with a different commercial anti-tTG assay. Available subjects with one or more positive assay results were interviewed, had serum collected for repeat anti-tTG assays and for HLA-DQ2 and HLA-DQ8 haplotyping and, if appropriate, gastroscopy and duodenal biopsy were performed. In unavailable subjects, HLA-DQ2 and -DQ8 haplotyping was performed on stored sera. Total serum IgA levels were assessed in subjects with initially negative assay results.
Main outcome measure: Prevalence of anti-tTG positivity and biopsy-proven CD.
Results: In 47 of 3011 serum samples (1.56%), at least one anti-tTG assay gave positive results: 31 of the subjects who provided these sera were available for clinical review, and 21 were able to have a gastroscopy. Seventeen subjects (0.56%) were diagnosed with definite CD (14 were confirmed at gastroscopy, and three unavailable subjects had three positive results of anti-tTG assays and an HLA haplotype consistent with CD); in a further 12 unavailable subjects, CD status was considered equivocal, with one or more positive anti-tTG assay results and an HLA haplotype consistent with CD. If these subjects were regarded as having CD, the prevalence of CD would be 0.96%. The positive predictive value when all three anti-tTG assays gave positive results was 94%, but fell to 45.2% with only one positive result.
Conclusions: The prevalence of anti-tTG antibodies in this population is 1.56%; the prevalence of CD is at least 0.56%. The utility of a single, positive result of an anti-tTG assay in screening for CD in the community is poor, and repeat and/or collateral assessment with different assays may decrease the need for gastroscopy and distal duodenal biopsy.