A 49-year-old health care worker received varicella vaccine in accordance with current Australian guidelines. She developed streptococcal toxic shock syndrome, complicated by acute atraumatic dislocation of the right wrist secondary to poststreptococcal reactive arthritis — to our knowledge, the first report of spontaneous wrist dislocation as a complication in this condition. Vaccination was accompanied by prolonged viraemia with the varicella vaccine strain — also, we believe, the first report of this in an immunocompetent patient.
A 49-year-old female hospital employee received varicella vaccination, in accordance with the guidelines of New South Wales Health for varicella-seronegative health care workers.1 She presented 17 days after the second vaccine dose (38 days after the first dose) with a 12-day history of joint pain and swelling, predominantly affecting the upper limbs and knees, along with myalgia and lethargy. These symptoms had worsened over the preceding 48 hours. There were no noticeable skin lesions, and no local reaction at the vaccination site.
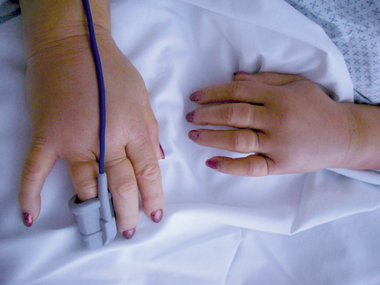
At presentation, she was afebrile, with a blood pressure of 95/60 mmHg, and pulse rate of 96 beats/min. The dominant clinical finding was gross peripheral oedema of the upper limbs, including the hands (Box 1).
Varicella zoster virus (VZV) genotyping of serum showed the presence of the Oka vaccine strain of VZV.2 Nucleic acid testing by quantitative polymerase chain reaction (PCR) using primers that target VZV open reading frame 62 revealed a serum load of 480 000 VZV DNA copies/mL 2 days after admission and 19 days after the last varicella vaccination. Tests on admission for VZV-specific IgG were positive.
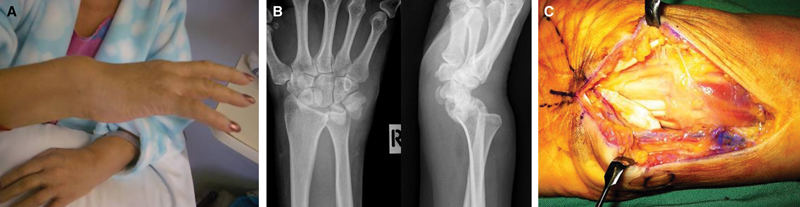
Nineteen days after admission, the patient showed signs of bilateral median nerve compression. The right wrist appeared clinically deformed, with palpable synovitis (Box 2A ). Imaging showed disruption of the right wrist and carpus to a degree usually associated with high-energy trauma (in the absence of any history of trauma), with dislocation of the distal radioulnar joint (DRUJ), and wide diastasis of the scapholunate interval (Box 2B). The left wrist and carpus showed lesser disruption.
The following day, the patient underwent aspiration of the left knee effusion, and bilateral carpal tunnel decompression and flexor synovectomy. Extensive synovitis was observed around the flexor tendons of both wrists (Box 2C), with rupture of the right lunotriquetral ligament. The right DRUJ was reduced and held in a supination splint, avoiding the insertion of metalware until infection had been excluded.
Varicella vaccine contains live attenuated virus (Oka/Merck strains), and has been used in Australia since 2000.3 Although the Oka vaccine strain has been detected in patients with varicella or zoster-like rashes after VZV vaccination,4 its persistence and load in blood after vaccination of immunocompetent adults has not been established. In a study of primary varicella infection, wild-type virus was not detected more than 8 days after the onset of rash, nor in any patient who received aciclovir.5 Another study was unable to detect virus more than 14 days after onset of illness.6 In contrast, in our patient, viraemia persisted for 54 days and the Oka VZV strain was detectable after aciclovir treatment.
S. pyogenes infection has long been recognised as a sequelae of chickenpox.7-9 A study found that up to 50% of cases of invasive group A streptococcal infections in children were associated with recent VZV infection.7 However, in previous cases, varicella infection was clearly apparent, and skin lesions were present from which secondary bacterial infection was presumed to arise. Our patient had no clinically apparent chickenpox-like skin lesions, and, in her case, the association between the varicella vaccination and streptococcal infection cannot be clearly defined.
The frequency of poststreptococcal reactive arthritis complicating streptococcal septicaemia is difficult to determine because of the heterogeneity of the condition and lack of well accepted diagnostic criteria.10 Although palmar flexor tenosynovitis is described,11 acute atraumatic wrist dislocation has not been documented previously as a complication of poststreptococcal reactive arthritis. Occasional reports of atraumatic dislocation of the wrist have been in the setting of a pre-existing connective tissue disorder or had unknown aetiology.12,13
The M11 serotype of S. pyogenes isolated from our patient has been reported previously in invasive streptococcal disease, although M1 and M3 are the most commonly isolated serotypes.8 However, we believe this is the first report of the M11 serotype as a cause of poststreptococcal reactive arthritis,14 possibly reflecting a more virulent strain.
This case highlights the possibility of prolonged high-level viraemia following varicella vaccination and the possible association with invasive S. pyogenes disease. However, this must be considered in the context of the benefits of varicella vaccination in preventing transmission of disease to health care workers15 and susceptible individuals.
- Claire M Italiano1
- Cheryl S Toi2
- Simon P Chan3
- Dominic E Dwyer4
- Westmead Hospital, Sydney, NSW.
- 1. Occupational assessment, screening and vaccination against specified infectious diseases. NSW Health Policy Directive 1 Feb 2007.
- 2. Toi CS, Dwyer DE. Differentiation between vaccine and wild-type varicella-zoster virus genotypes by high-resolution melt analysis of single nucleotide polymorphisms. J Clin Virol 2008; 43: 18-24.
- 3. Uahwatanasakul W, Carapetis JR. Frequently asked questions about varicella vaccine. Aust Prescriber 2005; 28: 2-5.
- 4. Sharrar RG, LaRussa P, Galea SA, et al. The postmarketing safety profile of varicella vaccine. Vaccine 2000; 19: 916-923.
- 5. Mainka CB, Fuss B, Geiger H, et al. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol 1998; 56: 91-98.
- 6. Kimura H, Kido S, Ozaki T, et al. Comparison of quantitations of viral load in varicella and zoster. J Clin Microbiol 2000; 38: 2447-2449.
- 7. Doctor A, Harper MB, Fleisher GR. Group A β-haemolytic streptococcal bacteremia: historical overview, changing incidence, and recent association with varicella. Pediatrics 1995; 96: 428-433.
- 8. Kiska DL, Thiede B, Caracciolo J, et al. Invasive Group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J Infect Dis 1997; 176: 992-1000.
- 9. Stride PJO, Coulter C, Campher MJJ, et al. Adult chickenpox complicated by fatal necrotising pneumonia. Med J Aust 2004; 181: 160-161. <MJA full text>
- 10. Mackie SL, Keat A. Poststreptococcal reactive arthritis: what is it and how do we know? Rheumatology 2004; 43: 949-954.
- 11. Gutiérrez-Ureña S, Molina J, Molina JF, et al. Poststreptococcal reactive arthritis, clinical course, and outcome in 6 adult patients. J Rheumatol 1995; 22: 1710-1713.
- 12. Dabbas N, Saker R, Blakely C. Multiple spontaneous dislocations in a patient with Ehlers-Danlos syndrome. Emerg Med J 2008; 25: 175-176.
- 13. Stokes AC. Spontaneous forward dislocation of wrist-joint. (Madelung’s deformity). Ann Surg 1910; 52: 229-238.
- 14. Jansen TLTA, Janssen M, Traksel R, de Jong AJL. A clinical and serological comparison of group A versus non-group A streptococcal reactive arthritis and throat culture negative cases of poststreptococcal reactive arthritis. Ann Rheum Dis 1999; 58: 410-414.
- 15. Rice P, Shin GY, Mitchell-Heggs N. Varicella zoster vaccination of health care workers is cost-effective. J Clin Virol 2006; 36: S46.