Cardiogenic shock (CS) is a common complication of acute myocardial infarction (AMI), occurring in 5%–10% of patients with an ST-segment-elevation myocardial infarction (STEMI).1 Up to half of patients admitted to hospital with CS will not survive to discharge.2
There have been substantial improvements in survival among patients with CS since the 1980s3,4 — a pattern paralleled by the increased use of emergency coronary revascularisation.4 In the United States, survival benefits have been realised in tertiary hospitals, but survival in regional or community hospitals remains poor.5 A similar reduction in overall mortality from CS has been apparent in Australia during the 10 years 1995–2004 (unpublished data from the Australian and New Zealand Intensive Care Society database).
Despite evidence that percutaneous coronary intervention (PCI) outperforms thrombolysis as a means of reperfusion after AMI,6 non-tertiary centres still play an important role in managing acute coronary syndromes.7,8 This role is likely to be emphasised in countries with a low-density popu-lation and dispersed PCI facilities, such as Australia. Further, despite evidence that non-interventional management of CS worsens prognosis,9,10 patients with CS frequently present to regional hospitals. Indeed, 69% of patients with CS admitted to the intensive care unit of our tertiary cardiothoracic hospital between 2000 and 2007 were referred from non-tertiary centres (unpublished data).
We summarised relevant studies and graded their evidence according to National Health and Medical Research Council (NHMRC) guidelines.11 Results were tabulated and presented as forest plots. Odds ratios of death were calculated using the Mantel–Haenszel method.
One-hundred and 15 articles were identified for possible inclusion, from which 35 were selected for detailed review. These included four meta-analyses,12-15 eight randomised controlled trials2,16-22 and 23 cohort studies.23-45
After STEMI, 71%–89% of patients who develop CS do so after being admitted to hospital.4,23,24 Therefore, in the absence of onsite revascularisation facilities, these patients should receive IHT based on their clinical presentation and electrocardiogram changes.8 IHT reduces the incidence of CS complicating AMI, an effect which may favour use of tissue-specific thrombolytic agents.46,47
The use of IHT in patients with STEMI and established CS is more controversial. Large multicentre randomised controlled trials of thrombolysis have either had few patients with CS at baseline48,49 or did not consistently report the incidence of baseline CS.24,47,50-52
A retrospective cohort study evaluated IHT and IABP in patients with STEMI and CS enrolled in the SHOCK (SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK) trial and registry.25 Patients who were treated with IHT had lower inhospital mortality than those who were not (54% v 64%; P = 0.005; odds ratio [OR], 0.66). Baseline characteristics were poorly matched between groups.
In a subsequent analysis of the SHOCK trial,26 patients assigned to medical therapy who received thrombolysis had a higher 12-month survival rate than those without thrombolysis (64% v 37%; mortality hazard ratio, 0.59; P = 0.01).
In a trial of indications for fibrinolytic therapy in suspected AMI, pooled data from nine randomised controlled trials with 58 600 patients were used to evaluate thrombolysis in patients with AMI.12 When patients were stratified according to presenting systolic blood pressure, mortality was highest in those with systolic blood pressure of over 100 mmHg (2445 patients). Thrombolysis in this group improved 35-day survival from 64.9% to 71.1% (P < 0.001). However, the control group mortality of 35.1% is better than would be expected for CS, and suggests that these patients may not all have had an accurate diagnosis of CS.
Patients with AMI who satisfy clinical and electrocardiographic criteria should receive thrombolysis (level I).
Current evidence favours the use of IHT in the management of eligible patients with STEMI and systolic blood pressure of over 100 mmHg (level I) and patients with STEMI and CS (level III-2).
One meta-analysis,13 three randomised controlled trials16-18 and three cohort studies27-29 were included. In non-tertiary hospitals, PHT may be most suitable as a substitute for IHT, and multiple studies have evaluated its role in this setting. A meta-analysis of six randomised trials including 6434 patients with STEMI that compared PHT and IHT reported a significantly reduced all-cause mortality with PHT use (OR, 0.83; 95% CI, 0.70–0.98). Median time from symptom onset to PHT or IHT was 104 minutes and 162 minutes, respectively.13 The incidence of CS was not significantly reduced with PHT, but only two studies in the meta-analysis reported this secondary outcome.16,17 Since then, three further studies have shown a mortality benefit and a significant reduction in CS with PHT compared with IHT.27-29
In the CAPTIM (Comparaison de l’Angioplastie Primaire et de la Thrombolyse préhospitalière à la phase aiguë de l’Infarctus du Myocarde) trial (the only randomised controlled trial of PCI versus PHT),18 840 patients were randomly assigned to PHT with alteplase or to immediate coronary angiography and revascularisation, as indicated. The composite primary endpoint of 30-day death, non-fatal reinfarction and non-disabling stroke occurred in 8.2% of the PHT group and 6.2% of the PCI group (P = 0.29). PHT significantly reduced the risk of CS on arrival at hospital. On further analysis, both 30-day mortality and CS were reduced in patients given PHT within 2 hours of symptom onset.53 Finally, a recent prospective cohort study of PHT versus IHT reported a significant reduction in CS following STEMI in patients treated with PHT (6.8% v 11.5%; P < 0.001).28
After STEMI, PHT is more effective than IHT in reducing all-cause mortality (level I) and is not inferior to PCI (level II).
PHT consistently reduces the time to drug administration in patients with STEMI (level I).
PHT reduces the incidence of CS after STEMI (level III-2).
PHT administered within 2 hours of symptom onset may reduce mortality and CS after STEMI, compared with PCI (level III-2).
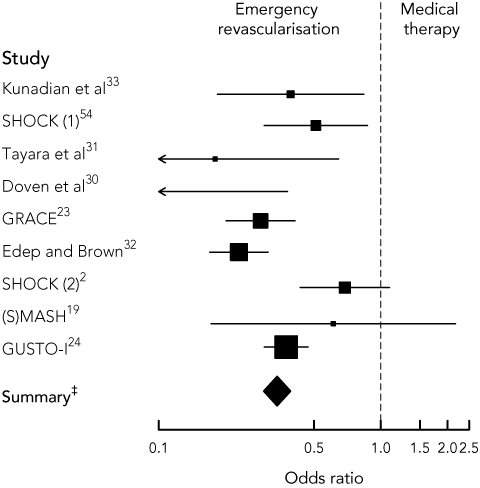
Two randomised controlled trials2,19 and six cohort studies23,24,30-33 evaluated ERV in the management of CS. These studies are summarised in Box 1. The use of ERV significantly reduced mortality from CS relative to medical therapy (pooled OR, 0.34; 95% CI, 0.30–0.39).
In the landmark SHOCK trial,2 302 patients with STEMI and CS were randomly assigned to receive ERV (PCI or coronary artery bypass grafting) or medical therapy. ERV conferred a mortality benefit at 6 months, 1 year and 6 years. The significance of this study is its relevance to practices in non-tertiary hospitals. Fifty-five per cent of patients in the intervention group were transferred from other hospitals to receive revascularisation. The (S)MASH ([Swiss] Multicenter trial of Angioplasty for SHock) trial,19 a study of invasive versus medical therapy for CS, was terminated because of problems with patient recruitment. Of those randomly assigned, patients treated invasively had a non-significant reduction in resolution of CS and 30-day mortality.
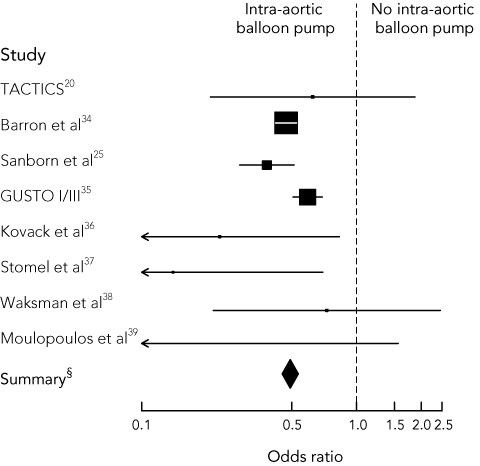
The IABP uses timed balloon inflation and deflation in the upper descending aorta to augment coronary perfusion during diastole, and to reduce myocardial oxygen demand during systole. We reviewed one randomised controlled trial20 and seven cohort studies25,34-39 of IABP use in CS. The results of these studies are summarised in Box 2. The addition of IABP therapy to the management of CS significantly reduced the risk of death (OR, 0.49; 95% CI, 0.45–0.53).
The Thrombolysis And Counterpulsation To Improve Cardiogenic Shock survival (TACTICS) trial enrolled patients with STEMI and CS who presented to a non-tertiary hospital.20 After thrombolysis, patients were randomly assigned to 48 hours of IABP or standard therapy. Although there was no difference in mortality in all patients, IABP use in Killip class III/IV patients significantly improved 6-month survival (61% v 20%; P = 0.05).55
As summarised in Box 2, most published observational data report a treatment benefit with IABP use in CS, although these findings may reflect patient selection bias or publication bias.56 Despite more frequent use of IABP in tertiary cardiac hospitals, two small studies have demonstrated the benefit of IABP in the management of CS in the community hospital setting.36,37 Moreover, in the SHOCK trial, 55% of the intervention group were transferred from a non-specialist centre for mechanical revascularisation, and 86% had IABP insertion.2
In the management of STEMI and CS, IABP use should be considered in all patients who do not have contraindications for insertion (level III-2).
IABP insertion in the non-tertiary hospital setting is feasible, confers a survival benefit and facilitates transfer to a tertiary centre for definitive therapy (level III-2).
Only two randomised controlled trials evaluated the early administration of GP inhibitors and reported prevention of CS.21,22 There were no randomised trials evaluating GP inhibition in established CS. The addition of GP IIb/IIIa inhibitors to thrombolytic therapy offers no advantage over thrombolysis alone in the prevention of CS. The GUSTO-V trial randomly allocated 16 588 patients within 6 hours of onset of STEMI to receive reteplase or half dose reteplase and abciximab.21 Despite more bleeding complications with combined therapy, abciximab did not alter mortality nor reduce the risk of CS (9.1% in the intervention [combined therapy] group versus 9.4% in the control [reteplase alone] group). Recent findings from the FINESSE (Facilitated INtervention with Enhanced reperfusion Speed to Stop Events) study show that early use of GP IIb/IIIa inhibitors to facilitate PCI does not reduce mortality or CS, but does increase the risk of major and minor bleeding.22
There is a lack of original research specifically evaluating treatments for AMI or CS in older patients, and many large randomised controlled trials excluded such patients from enrolment. However, consensus guidelines for acute coronary care in older patients have been published.57 Advanced age is a predictor of poor outcome in patients with STEMI, both with and without CS.24,58
Thrombolysis: Three meta-analyses12,14,15 and five observational studies40-44 were included. An overview of randomised trials evaluating thrombolysis against open or placebo control by the Fibrinolytic Therapy Trialists’ Collaborative Group included nine trials.12 The overall survival benefit from thrombolysis was not apparent in 5788 patients aged 75 years or older. However, as some of the included trials enrolled patients with ST-segment depression, a subsequent analysis of the 3300 patients aged 75 years or older who presented with STEMI or new left bundle branch block showed a significant reduction in mortality with thrombolytic therapy (26% v 29.4%; P = 0.03).14
A more recent meta-analysis reported a fourfold increase in mortality in older patients (aged ≥ 75 years) with AMI receiving thrombolysis compared with younger patients (aged < 75 years).15 Despite a threefold increase in intracranial haemorrhage and non-haemorrhagic cerebrovascular accident after thrombolysis, the absolute incidence of these complications was low (intracranial haemorrhage, 1.4%; cerebrovascular accident, 3.5%) and older patients were more likely to die from complications of AMI than from thrombolysis.
Observational data do not universally endorse fibrinolysis in older patients,40,41 but data from large registries and consensus opinion support its use in the appropriate clinical setting.42-44,57
ERV: Box 3 summarises the studies of ERV in older patients with CS. The only randomised data are either from an underpowered study19 or a subgroup analysis.2 The remaining study was a retrospective cohort study.45
IABP: The only randomised trial of IABP use in STEMI and CS sought to enrol patients aged over 75 years, but the oldest patient was aged 74 years.20 In the SHOCK trial, 82% of patients aged over 75 years had IABP support, but 1-year mortality was shown to be unchanged in the IABP group.2 In non-randomised studies of IABP in CS, the mean age of patients who underwent IABP therapy was consistently 62–67 years.34-39
Thrombolysis:
Patients aged 75 years or older who have STEMI are more likely to die from complications of AMI than from complications of thrombolysis (level I).
In older patients with AMI, thrombolysis reduces all-cause mortality compared with no reperfusion therapy (level I).
ERV:
While observational data support the use of ERV in patients aged 75 years or older who have CS (level III-2), this is not matched by the findings of subgroup analysis from a randomised trial.
IABP:
There is no evidence to support the isolated use of IABP in older patients with CS.
Despite conflicting evidence, selected older patients with CS may benefit from thrombolysis and invasive therapies such as ERV and IABP.
Despite reductions in mortality from AMI in the past 20 years, the rate of survival among patients who develop CS is less than 50% at 1 year.2 Although the mortality rate from CS is higher among patients presenting to non-tertiary hospitals than to hospitals with invasive cardiac facilities, CS is frequently managed initially in regional centres. In this review, we sought to identify treatments that may reduce the incidence of or improve outcomes from CS and that could be easily initiated in the community hospital setting. While PCI outperforms thrombolysis for revascularisation in CS, it is difficult to achieve a timely PCI service for all patients in a large country like Australia. Thrombolytic therapy for STEMI reduces all-cause mortality and reduces the incidence of CS. PHT confers greater benefit than IHT and, if it is delivered within 2 hours of symptom onset, may reduce CS and mortality to a greater degree than PCI. In patients aged less than 75 years who have STEMI and CS, thrombolysis should be administered in keeping with recognised indications and contraindications, referral should be made to a tertiary centre for consideration for ERV, and IABP should be contemplated before interhospital transfer. Although increasing age is an independent predictor of mortality in CS, patients aged 75 years or older should not be denied treatment on the basis of their age. Such patients with STEMI may benefit from thrombolysis and/or transfer for early invasive therapies, and the choice of management should be based on their functional status, comorbidities and the severity of their acute illness. There is no role for GP IIb/IIIa inhibitors in the prevention or management of CS in the community hospital setting.
1 Comparison of emergency revascularisation (ERV) with medical therapy in published studies of patients with ST-segment-elevation myocardial infarction and cardiogenic shock
Forest plot showing odds ratios (95% CIs) of death
2 Comparison of treatment with intra-aortic balloon pump (IABP) with no such treatment in published studies of patients with ST-segment-elevation myocardial infarction and cardiogenic shock
Forest plot showing odds ratios (95% CIs) of death
Received 22 June 2008, accepted 14 September 2008
- Enda O’Connor1
- John F Fraser2
- Prince Charles Hospital, Brisbane, QLD.
We thank Adrian Barnett, School of Public Health, Queensland University of Technology, Brisbane, for assistance with statistical calculations. We also thank Medtel Australia for their financial contribution to offset the costs of publishing this article (Medtel were not involved in article preparation at any stage).
John Fraser received travel assistance from Datascope, a manufacturer of intra-aortic balloon pumps, to speak at a meeting in Cairns in 2008, although he did not speak about their products.
- 1. Reynolds HR, Hochman JS. Cardiogenic shock, current concepts and improving outcomes. Circulation 2008; 117: 686-697.
- 2. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock? N Engl J Med 1999; 341: 625-634.
- 3. Goldberg RJ, Samad NA, Yarzebski J, et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999; 340: 1162-1168.
- 4. Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005; 294: 448-454.
- 5. Babaev A, Every N, Frederick P, et al. Trends in revascularisation and mortality in patients with cardiogenic shock complicating acute myocardial infarction. Observations from the national registry of myocardial infarction. Circulation 2002; 106 Suppl II: II-364.
- 6. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361: 13-20.
- 7. Rogers WJ, Canto JG, Barron HV, et al. Treatment and outcome of myocardial infarction in hospitals with and without invasive capability. Investigators in the National Registry of Myocardial Infarction. J Am Coll Cardiol 2000; 35: 371-379.
- 8. Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: a comprehensive review of contemporary management options. J Am Coll Cardiol 2007; 50: 917-929.
- 9. Barbash IM, Behar S, Battler A, et al. Management and outcome of cardiogenic shock complicating acute myocardial infarction in hospitals with and without on-site catheterisation facilities. Heart 2001; 86: 145-149.
- 10. Mayich J, Cox JL, Buth KJ, Legare JF. Unequal access to interventional cardiac care in Nova Scotia in patients with acute myocardial infarction complicated by cardiogenic shock. Can J Cardiol 2006; 22: 331-335.
- 11. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999. http://www.nhmrc.gov. au/publications/synopses/cp30syn.htm (accessed Mar 2009).
- 12. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343: 311-322.
- 13. Morrison LJ, Verbeek PR, McDonald AC, et al. Mortality and prehospital thrombolysis for acute myocardial infarction: a meta-analysis. JAMA 2000; 283: 2686-2692.
- 14. White HD. Thrombolytic therapy in the elderly. Lancet 2000; 356: 2028-2030.
- 15. Ahmed S, Antman EM, Murphy SA, et al. Poor outcomes after fibrinolytic therapy for ST-segment elevation myocardial infarction: impact of age (a meta-analysis of a decade of trials). J Thromb Thrombolysis 2006; 21: 119-129.
- 16. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. The European Myocardial Infarction Project Group. N Engl J Med 1993; 329: 383-389.
- 17. Feasibility, safety and efficacy of domiciliary thrombolysis by general practitioners: Grampian region early anistreplase trial. GREAT group. BMJ 1992; 305: 548-553.
- 18. Bonnefoy E, Lapostolle F, Leizorovicz A, et al. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet 2002; 360: 825-829.
- 19. Urban P, Stauffer JC, Bleed D, et al. A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction. The (Swiss) Multicenter Trial of Angioplasty for Shock-(S)MASH. Eur Heart J 1999; 20: 1030-1038.
- 20. Ohman EM, Nanas J, Stomel RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS trial. J Thromb Thrombolysis 2005; 19: 33-39.
- 21. Topol EJ; GUSTO V Investigators. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet 2001; 357: 1905-1914.
- 22. Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358: 2205-2217.
- 23. Dauerman HL, Goldberg RJ, White K, et al; GRACE Investigators. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol 2002; 90: 838-842.
- 24. Berger PB, Holmes DR Jr, Stebbins AL, et al. Impact of an aggressive invasive catheterization and revascularization strategy on mortality in patients with cardiogenic shock in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial. An observational study. Circulation 1997; 96: 122-127.
- 25. Sanborn TA, Sleeper LA, Bates ER, et al. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shock? J Am Coll Cardiol 2000; 36: 1123-1129.
- 26. French JK, Feldman HA, Assmann SF, et al. Influence of thrombolytic therapy, with or without intra-aortic balloon counterpulsation, on 12-month survival in the SHOCK trial. Am Heart J 2003; 146: 804-810.
- 27. Stenestrand U, Lindbäck J, Wallentin L. Long-term outcome of primary percutaneous coronary intervention vs prehospital and in-hospital thrombolysis for patients with ST-elevation myocardial infarction. JAMA 2006; 296: 1749-1756.
- 28. Björklund E, Stenestrand U, Lindbäck J, et al. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27: 1146-1152.
- 29. McAleer B, Varma MPS. Feasibility and long term outcome of home vs hospital initiated thrombolysis. Ir J Med Sci 2006; 175: 14-19.
- 30. Doven O, Akkus MN, Camsari A, et al. Impact of invasive strategy for the management of patients with cardiogenic shock after acute myocardial infarction. Coron Artery Dis 2004; 15: 361-366.
- 31. Tayara W, Starling RC, Yamani MH, et al. Improved survival after acute myocardial infarction complicated by cardiogenic shock with circulatory support and transplantation: comparing aggressive intervention with conservative treatment. J Heart Lung Transplant 2006; 25: 504-509.
- 32. Edep ME, Brown DL. Effect of early revascularization on mortality from cardiogenic shock complicating acute myocardial infarction in California. Am J Cardiol 2000; 85: 1185-1188.
- 33. Kunadian B, Vijayalakshmi K, Dunning J, et al. Should patients in cardiogenic shock undergo rescue angioplasty after failed fibrinolysis? Comparison of primary versus rescue angioplasty in cardiogenic shock patients. J Invasive Cardiol 2007; 19: 217-223.
- 34. Barron HV, Every NR, Parsons LS, et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J 2001; 141: 933-939.
- 35. Hudson MP, Granger CB, Stebbins AL, et al. Cardiogenic shock survival and use of intraaortic balloon counterpulsation: results from the GUSTO I and III trials. Circulation 1999; 100 Suppl I: I-370.
- 36. Kovack PJ, Rasak MA, Bates ER, et al. Thrombolysis plus aortic counterpulsation: improved survival in patients who present to community hospitals with cardiogenic shock. J Am Coll Cardiol 1997; 29: 1454-1458.
- 37. Stomel RJ, Rasak M, Bates ER. Treatment strategies for acute myocardial infarction complicated by cardiogenic shock in a community hospital. Chest 1994; 105: 997-1002.
- 38. Waksman R, Weiss AT, Gotsman MS, Hasin Y. Intra-aortic balloon counterpulsation improves survival in cardiogenic shock complicating acute myocardial infarction. Eur Heart J 1993; 14: 71-74.
- 39. Moulopoulos S, Stamatelopoulos S, Petrou P. Intraaortic balloon assistance in intractable cardiogenic shock. Eur Heart J 1986; 7: 396-403.
- 40. Soumerai SB, McLaughlin TJ, Ross-Degnan D, et al. Effectiveness of thrombolytic therapy for acute myocardial infarction in the elderly: cause for concern in the old-old. Arch Intern Med 2002; 162: 561-568.
- 41. Thiemann DR, Coresh J, Schulman SP, et al. Lack of benefit for intravenous thrombolysis in patients with myocardial infarction who are older than 75 years. Circulation 2000; 101: 2239-2246.
- 42. Angeja BG, Rundle AC, Gurwitz JH, et al. Death or nonfatal stroke in patients with acute myocardial infarction treated with tissue plasminogen activator. Participants in the National Registry of Myocardial Infarction 2. Am J Cardiol 2001; 87: 627-630.
- 43. Berger AK, Radford MJ, Wang Y, Krumholz HM. Thrombolytic therapy in older patients. J Am Coll Cardiol 2000; 36: 366-374.
- 44. Stenestrand U, Wallentin L; Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163: 965-971.
- 45. Dzavik V, Sleeper LA, Cocke TP, et al. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: a report from the SHOCK Trial Registry. Eur Heart J 2003; 24: 828-837.
- 46. Wilcox RG, von der Lippe G, Olsson CG, et al. Trial of tissue plasminogen activator for mortality reduction in acute myocardial infarction. Anglo-Scandinavian Study of Early Thrombolysis (ASSET). Lancet 1988; 2: 525-530.
- 47. Neuhaus KL, von Essen R, Tebbe U, et al. Improved thrombolysis in acute myocardial infarction with front-loaded administration of alteplase: results of the rt-PA-APSAC patency study (TAPS). J Am Coll Cardiol 1992; 19: 885-891.
- 48. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41,299 cases of suspected acute myocardial infarction. ISIS-3 (Third International Study of Infarct Survival) Collaborative Group. Lancet 1992; 339: 753-770.
- 49. A comparison of reteplase with alteplase for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. N Engl J Med 1997; 337: 1118-1123.
- 50. Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. International Joint Efficacy Comparison of Thrombolytics. Lancet 1995; 346: 329-336.
- 51. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988; 2: 349-360.
- 52. Effect of intravenous APSAC on mortality after acute myocardial infarction: preliminary report of a placebo-controlled clinical trial. AIMS Trial Study Group. Lancet 1988; 1: 545-549.
- 53. Steg PG, Bonnefoy E, Chabaud S, et al. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108: 2851-2856.
- 54. Hochman JS, Sleeper LA, Webb JG, et al; SHOCK Investigators. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 2006; 295: 2511-2515.
- 55. Killip T, Kimball JT. Treatment of myocardial infarction in the coronary care unit: a 2 year experience of 250 patients. Am J Cardiol 1967; 20: 457-464.
- 56. Ohman EM, Hochman JS. Aortic counterpulsation in acute myocardial infarction: physiologically important but does the patient benefit? Am Heart J 2001; 141: 889-892.
- 57. Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation 2007; 115: 2570-2589.
- 58. Singh M, White J, Hasdai D, et al. Long-term outcome and its predictors among patients with ST-segment elevation myocardial infarction complicated by shock: insights from the GUSTO-I trial. J Am Coll Cardiol 2007; 50: 1752-1758.





Abstract
Objective: To evaluate current evidence in support of therapies for preventing and treating cardiogenic shock (CS) after acute myocardial infarction that can be initiated in hospitals without invasive cardiac facilities.
Study design: Systematic review.
Data sources: MEDLINE and PubMed were searched from January 1985 to May 2008 using the MeSH terms “myocardial infarction”, “thrombolytic therapy”, “shock, cardiogenic”, “angioplasty, transluminal, percutaneous coronary”, “intra-aortic balloon pumping” and “platelet aggregation inhibitors”. Additional keyword and reference list searches were performed. Articles in English relating to adults were included.
Study selection: Meta-analyses and comparative studies were included if they reported mortality or prevention of CS as an endpoint. In total, 35 articles were analysed (four meta-analyses, eight randomised controlled trials and 23 cohort studies).
Data extraction: Studies were summarised by the first author and the level of evidence graded. Each study was checked by the second author and consensus was reached about inclusion and levels of evidence.
Data synthesis: In the management and prevention of CS, the following are supported by high-level evidence: prehospital thrombolysis, transfer for emergency revascularisation (patients aged < 75 years) and thrombolysis for older patients (patients aged ≥ 75 years). In established CS, evidence supporting inhospital thrombolysis and intra-aortic balloon pump use in patients aged < 75 years and emergency revascularisation in older patients is limited to subgroup analyses and observational studies.
Conclusions: In regional centres, prevention of CS is achieved with early fibrinolysis, preferably before hospital arrival. Patients of all ages should be considered for thrombolysis, early transfer for coronary revascularisation, and intra-aortic balloon pump insertion unless contraindicated. Glycoprotein inhibitors have no role in the management of CS in non-tertiary hospitals.