A 32-year-old woman with a 4-year history of multiple sclerosis presented with persistent clawing of the right hand. History revealed that she and five family members had lifelong symptoms of paradoxical myotonia (impaired relaxation of muscles following muscle contraction), exacerbated by cold. The family was diagnosed with paramyotonia congenita, based on neurophysiological and genetic studies. To our knowledge, this is the first report of an Australian family with paramyotonia congenita.
Clinical record
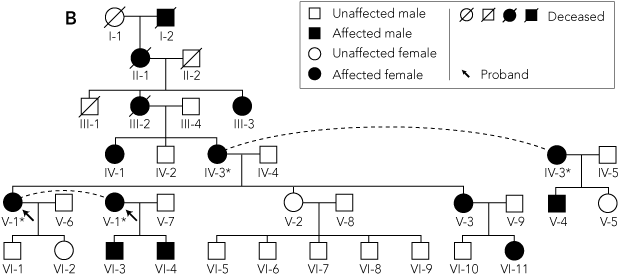 | |||||||||||||||
* Individuals IV-3 and V-1 each appear twice in the pedigree, as indicated by the dashed lines. | |||||||||||||||
To our knowledge, this is the first report of an Australian family with paramyotonia congenita. Paramyotonia congenita is a rare autosomal dominant condition characterised by paradoxical myotonia — impaired relaxation of muscles following muscle contraction, which worsens with repetitive muscle activity.1 It is distinct from other forms of myotonia, which typically improve with repetitive activity (the warm-up phenomenon).4 Paramyotonia is typically exacerbated by exposure to cold, and can be associated with cold-induced paralysis.1 Mutations in the skeletal muscle voltage-gated sodium channel gene SCN4A cause paramyotonia congenita,5 and can also cause hyperkalaemic periodic paralysis, potassium-aggravated myotonia, and a small proportion of hypokalaemic periodic paralysis cases.6 Patients carrying an SCN4A mutation may have manifestations of more than one of these allelic disorders.
Our patient’s neurophysiology findings and the clinical features of her affected family members are typical for paramyotonia congenita associated with cold paralysis, and similar to those described in the largest reported case series for this condition.7 In this case series, age of onset was typically during early childhood, and clinical myotonia was evident in 100% of the 56 patients studied. Cold was identified as a precipitant in 91% of patients, and exercise was identified as a precipitant in 46%. Electromyographic evidence for myotonia was seen in 100% of the patients, and 92% had a reduction in compound muscle action potential in response to cold (objective cold paralysis). Forty-nine of the patients (88%) had a mutation of the SCN4A gene. The electromyographic changes evident with cooling in our patient were typical for paramyotonia congenita — cooling initially resulted in abolition of the myotonic discharges, and electrical silence was recorded at lower temperatures.1
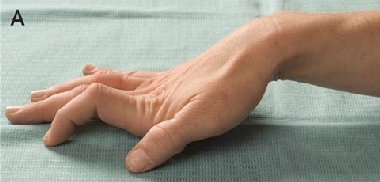
The T1313M mutation was identified in our patient and the five affected family members, but not an unaffected family member. This is one of the more common mutations that causes paramyotonia congenita,4,8,9 and has been reported to exclusively cause paramyotonia with the cold-paralysis phenotype.9-13 The hands and face are predominantly affected in patients who carry the T1313M mutation.9-13 A report of French families with paramyotonia congenita noted significant clinical variability in patients carrying the T1313M mutation — in both severity of myotonia and its permanence — and myotonia permanens was evident in six of eight of these patients.9 In our patient, the clawing of the right hand is also likely to reflect myotonia permanens, rather than an interaction between the paramyotonia and multiple sclerosis.
The T1313M mutation has been predominantly described in families with French ancestry, and to a lesser extent in families of English and Japanese background. 5,9,11 One de-novo mutation has been identified in the literature, which occurred in a Japanese man.12 All individuals from the six generations of the family we studied were born in Australia and lived in Australia, but the ancestral origins of the family are unknown.
Present knowledge of SCN4A channel physiology provides insight into the clinical manifestations seen in our patient and the affected members of her family. Voltage-gated sodium channels are heteromultimeric, integral membrane proteins; they are composed of a single large pore-forming α subunit, and 1 or 2 smaller β units.6 There are nine subtypes of α subunit, one of which is expressed in skeletal muscle — SCN4A.6 The α subunit is composed of four structurally homologous domains (D1–D4), with six membrane spanning segments (S1–S6) within each domain.6 The sodium channel is important for generating and propagating action potentials and switches through three functional states: activation (the open-channel state), inactivation, and recovery from inactivation.6
The consequences of the T1313M mutation have been studied by patch-clamp studies using various cell lines. 10,14-17 Overall, the mutation has been shown to slow the rate of channel inactivation, diminish the voltage dependence of inactivation, and increase the rate of recovery from inactivation. 10,14-17 These effects result in increased sodium conductance and prolongation of action potentials, which causes persistent activation of potassium channels, leading to relatively high levels of extracellular potassium.6,10,14-17 Elevated extracellular potassium levels increase the likelihood of afterdepolarisations, which may lead to action potentials on adjacent surface membranes.6 This can result in ongoing muscle contraction and slowed relaxation — the features of paramyotonia.6
Threonine-1313 is a highly conserved residue in the D3–D4 linker; it is important for channel inactivation, and thought to occlude the cytoplasmic portion of the channel.16 The change from the polar hydrophilic threonine to the larger non-polar methionine is thought to impair occlusion of the channel, and therefore impair inactivation.13,16 Although the effects of the mutation have been shown to be potentiated at lower temperatures, as might be expected, no study has shown an increased sensitivity to temperature compared with normal cells.15-17
Investigations
Biochemistry and neurophysiology
The patient’s serum potassium level was 4.2 mmol/L (reference range [RR], 3.2–4.3 mmol/L) and her creatine kinase level was slightly elevated at 191 U/L (RR, < 150 U/L). Routine nerve conduction study results were normal. Electromyography of the left abductor pollicis brevis and flexor digitorum superficialis revealed electrical myotonia and dense fibrillations. Cooling to below 28°C abolished the myotonic discharges and cooling below 22°C resulted in abolition of all spontaneous activity. The cooling test described by Streib1 was also performed. Before cooling, the amplitude of the left median compound muscle action potential recording over the abductor pollicis brevis muscle was 16.7 mV. The arm was then cooled to below 20°C by immersion in an ice bath and then rewarmed to 32°C using heat packs. The compound muscle action potential after rewarming was 4.5 mV (a 73% decrease).
Venous blood was then obtained from five family members who had symptoms that were similar to those of the patient (Figure, B; IV-1, IV-3, V-3, V-4, VI-4), as well as an unaffected family member (the patient’s half-sister), and mutational analysis was carried out. DNA was extracted by a proteinase K digestion method2 and amplified using the polymerase chain reaction (PCR) (forward primer sequence, 5'-TGGAGGCAGGAAGGGGAACT-3'; reverse primer sequence, 5'-GGCAGCACACACAGGACAGG-3'). The cycling conditions were: 3 min at 94°C × 1; and 30 s at 94°C, 40 s at 57°C, 1 min at 72°C × 30. The PCR reaction mixture contained 100 ng DNA, 0.5 μM of each primer, 80 μM of each deoxynucleoside triphosphate, 20 μM Tris-HCl (pH, 8.5), 50 μM KCI, 1.5 mM MgCI and 0.5 U Taq Ti DNA polymerase (Fisher Biotech, Perth, Australia).
Amplified products were separated on 3% agarose gels. The separated fragments were diluted to 10 ng/μL per 100 base pairs and then sequenced by the dideoxy termination method,3 using BigDye Terminator v3.1 chemistry (Applied Biosystems, Foster City, Calif, USA) and the 3100 Genetic Analyzer (Applied Biosystems).
- 1. Streib EW. AAEE minimonograph #27: differential diagnosis of myotonic syndromes. Muscle Nerve 1987; 10: 603-615.
- 2. Zeviani M, Moraes CT, DiMauro S, et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 1988; 38: 1339-1346.
- 3. Sanger F, Nicklen S, Coulson A. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci U S A 1977; 74: 5463-5467.
- 4. Jurkat-Rott K, Lehmann-Horn F. Muscle channelopathies and critical points in functional and genetic studies. J Clin Invest 2005; 115: 2000-2009.
- 5. McClatchey A, Van den Bergh P, Pericak-Vance M, et al. Temperature-sensitive mutations in the III–IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell 1992; 68: 769-774.
- 6. George L. Inherited disorders of voltage gated sodium channels. J Clin Invest 2005; 115: 1990-1999.
- 7. Miller M, Dias da Silva M, Miller H, et al. Correlating phenotype and genotype in the periodic paralyses. Neurology 2004; 63: 1647-1655.
- 8. Ptacek L. The familial periodic paralyses and nondystrophic myotonias. Am J Med 1998; 105: 58-70.
- 9. Plassart E, Reboul J, Rime C, et al. Mutations in the muscle sodium channel gene (SCN4A) in 13 French families with hyperkalaemic periodic paralysis and paramyotonia congenita: phenotype to genotype correlations and demonstration of the predominance of two mutations. Eur J Hum Genet 1994; 2: 110-124.
- 10. Tahmoush A, Schaller K, Zhang P, et al. Muscle sodium channel inactivation defect in paramyotonia congenita with the thr1313met mutation. Neuromusc Disord 1994; 4: 447-454.
- 11. Yamada T, Ochi H, Hara H, et al. A skeletal muscle sodium channel mutation in a Japanese family with paramyotonia congenita. J Neurol Sci 1995; 133: 192-193.
- 12. Fukudome T, Izumoto H, Goto H, et al. Paramyotonia congenita due to a de novo mutation: a case report. Muscle Nerve 2003; 28: 232-235.
- 13. Bouhours M, Sternberg D, Davoine C, et al. Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans. J Physiol 2003; 554: 635-647.
- 14. Boulos P, Heiman-Patterson T, Alexander G, Tahmoush A. Patch clamp studies of the thr1313met mutant sodium channel causing paramyotonia congenita. Muscle Nerve 2000; 23: 1736-1747.
- 15. Dice M, Abbruzzese J, Wheeler J, et al. Temperature-sensitive mutants in paramyotonia congenita mutants R1448C and T1313M. Muscle Nerve 2004; 30: 277-288.
- 16. Hayward L, Brown R, Cannon S. Inactivation defects caused by myotonia-associated mutations in the sodium channel III-IV linker. J Gen Physiol 1996; 107: 559-576.
- 17. Yang N, Ji S, Zhou M, et al. Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Natl Acad Sci U S A 1994; 91: 12785-12789.





We thank the patient and her family for consenting to publication of this case report. Publication was also approved by the Flinders Clinical Research Ethics Committee.
None identified.