A previously well 2-year-old girl presented with acute respiratory distress. After multiple investigations she was diagnosed with spontaneous chylothorax, attributed to strenuous vomiting. To our knowledge, this is the second reported case of spontaneous chylothorax occurring after the neonatal period. (MJA 2009; 190: 262-264)
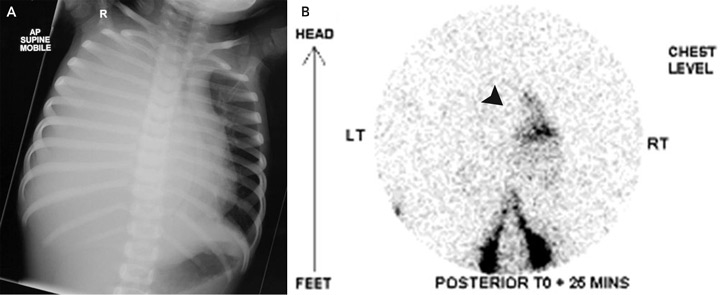
On examination, the patient’s temperature was 36.8°C, heart rate was 154 beats/min, respiratory rate was 67 breaths/min, and blood pressure was 96/68 mmHg. Her chest was dull to percussion, with poor air entry over the right hemithorax. The remainder of the physical examination was unremarkable. A chest x-ray (Box, A) revealed opacification of the right hemithorax with mediastinal shift to the left. An intercostal catheter (ICC) drained 900 mL of pink-stained milky fluid. Intravenous flucloxacillin and gentamicin therapy were begun for presumed empyema. She was transferred to a tertiary paediatric hospital.
Computed tomography of the chest excluded a mediastinal mass. Tests were negative for tumour markers, including α-fetoprotein, β-human chorionic gonadotropin and urinary biogenic amines. A Mantoux test returned a non-reactive result (diameter, 0 mm). Radionuclide lymphoscintigraphy (Box, B) confirmed normal lymphatic anatomy, but also detected rapid drainage of lymph into the right side of the chest.
This unusual case of chylothorax in a 2-year-old child had none of the previously recognised causes of chylothorax and was attributed to strenuous vomiting. To our knowledge, this is the second reported case of spontaneous chylothorax occurring after the neonatal period. Chylothorax is an uncommon cause of pleural effusion in children. Damage to the thoracic duct causes the pleural space to accumulate chyle — lymphatic fluid enriched with fat (chylomicrons) absorbed by the intestinal cells and transported into the circulation via the thoracic duct.1,2 It is well recognised in newborns as a congenital condition, or secondary to birth trauma. In childhood, it generally occurs after cardiac surgery. Other causes include neck surgery, scoliosis surgery, congenital malformations of the pulmonary or thoracic lymphatic system, and dysmorphic syndromes (Turner, Noonan and Down syndromes).2,3 Chylothorax can occur following blunt trauma to the chest, subclavian vein thrombosis or thoracic duct infiltration.
Our patient had no previously recognised causes of chylothorax. Multiple investigations were undertaken to identify a cause such as malformation, infiltration or injury to the thoracic duct. On review of the medical literature (MEDLINE search, 1956–2008), one reported case of spontaneous chylothorax in a child was identified.4 Straining of the thoracic duct due to strenuous vomiting was suggested to have caused the rupture, which we consider to be the likely explanation in our case. Despite appropriate medical management, the high rate of chyle leak necessitated surgical repair. The VATS approach is worth considering early in cases of spontaneous chylothorax when the chyle leak cannot be controlled with maximal medical therapy.
The diagnosis of chylothorax was straightforward in our patient. The fluid appeared milky, and the triglyceride level was extremely elevated, as was the lymphocyte count.1 The cause of the chylothorax was more difficult to ascertain. Knowledge of the anatomy of the thoracic lymphatic system is helpful in determining the site of disruption of the thoracic duct associated with right, left or bilateral chylothorax.
Rupture of the thoracic duct between the diaphragm and the fifth thoracic vertebra results in accumulation of chyle in the right pleural space. In adults, lymphography has been used to define the anatomy, but is not practical in children due to difficulty cannulating lymphatics; for this reason, we used radionuclide lymphoscintigraphy instead. This showed leakage of chyle above the diaphragm on the right side. Disruption of the thoracic duct at this point was confirmed by thoracoscopy. As both our patient and the previously reported patient4 had right-sided chylothorax, the thoracic duct might be most susceptible to injury from forceful diaphragmatic contraction as it traverses the diaphragm. Hence, forceful vomiting is the most likely explanation in our patient.
The management of chylothorax is the same regardless of cause, although no treatments have been subjected to a randomised controlled trial. The initial step is aspiration of pleural fluid for diagnosis. The basic principle of chylothorax management is to reduce the chyle flow in the thoracic duct while waiting for spontaneous healing. This is usually managed by a low-fat and MCT diet or, occasionally, enteric rest with total parenteral nutrition. Spontaneous healing can take weeks. MCT oil consists of triglycerides with saturated fatty acids that are 8–12 carbons in length; these are absorbed directly into the portal venous system, bypassing lymphatic drainage.5 A report on 51 children with chylothorax, aged 0–16 years (median age, 1.7 years), showed that most developed chylothorax secondary to cardiothoracic surgery (46/51), one did so after chest trauma, and four had congenital lymphatic malformation. Complete resolution of the chylothorax was achieved with a 4-week, low-fat and MCT diet in 80% of patients. Patients with congenital chylothorax or chylothorax secondary to obstruction were at higher risk of failure of medical treatment and proceeded to surgical repair.1
Octreotide, a somatostatin analogue, has recently been advocated for use in children with chylothorax that does not respond to conventional therapy.6,7 Somatostatin has a wide range of inhibitory effects on gastrointestinal and endocrine function. Its mechanism of action in treating chylothorax is unclear, but a possibility is reduction of splanchnic vascular tone, eventually leading to a decreased flow of chyle through the thoracic duct.8,9 Nevertheless, the efficacy of octreotide has not been demonstrated in a controlled trial. Although there is no clear dosage regimen, we used a continuous infusion as this was reported most often in the literature.10-13 Reduction in rate of chyle flow within 24–48 hours of treatment initiation has been reported, and the treatment appears to be safe.8,9,14
Some cases of chylothorax cannot be controlled by medical therapy, and surgery is required. There are numerous surgical approaches, including thoracic duct ligation and pleurodesis (surgical or chemical). VATS has been suggested recently, which is much less invasive than an open approach,15 and provides a superior view of the thoracic duct as it enters the thorax. In the previous report of spontaneous chylothorax in a child, ligation of the thoracic duct by VATS was also successful.4 There is no consensus on the timing of surgery, but most authors advocate 3–4 weeks of medical therapy beforehand.1,2,16 In our patient, surgery was undertaken earlier than this as spontaneous healing was considered very unlikely. Earlier surgery could reduce hospitalisation, malnutrition and risk of infection. Surgical correction is definitive and does not lead to lymph stasis because of the rich network of collateral lymphatic vessels.
Images used to diagnose a 2-year-old patient with spontaneous chylothorax
- 1. Beghetti M, La Scala G, Belli D, et al. Etiology and management of pediatric chylothorax. J Pediatr 2000; 136: 653-658.
- 2. Buttiker V, Fanconi S, Burger R. Chylothorax in children: guidelines for diagnosis and management. Chest 1999; 116: 682-687.
- 3. Bond SJ, Guzzetta PC, Snyder ML, Randolph JG. Management of pediatric postoperative chylothorax. Ann Thorac Surg 1993; 56: 469-472; discussion 472-473.
- 4. Achildi O, Smith BP, Grewal H. Thoracoscopic ligation of the thoracic duct in a child with spontaneous chylothorax. J Laparoendosc Adv Surg Tech A 2006; 16: 546-549.
- 5. Jensen GL, Mascioli EA, Meyer LP, et al. Dietary modification of chyle composition in chylothorax. Gastroenterology 1989; 97: 761-765.
- 6. Roehr CC, Jung A, Proquitte H, et al. Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review. Intensive Care Med 2006; 32: 650-657.
- 7. Chan SY, Lau W, Wong WH, et al. Chylothorax in children after congenital heart surgery. Ann Thorac Surg 2006; 82: 1650-1656.
- 8. Demos NJ, Kozel J, Scerbo JE. Somatostatin in the treatment of chylothorax. Chest 2001; 119: 964-966.
- 9. Cannizzaro V, Frey B, Bernet-Buettiker V. The role of somatostatin in the treatment of persistent chylothorax in children. Eur J Cardiothorac Surg 2006; 30: 49-53.
- 10. Lam JC, Aters S, Tobias JD. Initial experience with octreotide in the pediatric population. Am J Ther 2001; 8: 409-415.
- 11. Helin RD, Angeles ST, Bhat R. Octreotide therapy for chylothorax in infants and children: a brief review. Pediatr Crit Care Med 2006; 7: 576-579.
- 12. Landvoigt MT, Mullett CJ. Octreotide efficacy in the treatment of chylothoraces following cardiac surgery in infants and children. Pediatr Crit Care Med 2006; 7: 245-248.
- 13. Rosti L, De Battisti F, Butera G, et al. Octreotide in the management of postoperative chylothorax. Pediatr Cardiol 2005; 26: 440-443.
- 14. Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med 2006; 12: 264-267.
- 15. Graham DD, McGahren ED, Tribble CG, et al. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg 1994; 57: 1507-1511; discussion 1511-1512.
- 16. Allen EM, van Heeckeren DW, Spector ML, Blumer JL. Management of nutritional and infectious complications of postoperative chylothorax in children. J Pediatr Surg 1991; 26: 1169-1174.





None identified.