Reproductive ageing is an important social and medical development, and increased age is a very important factor in infertility (Box 1). The median age of all Australian women delivering babies (30 years in 2005) rose each year in the decade leading up to 2005, and 20.4% of all deliveries are now to mothers aged over 35 years. One in seven first-time mothers in 2005 (13.3%) were aged over 35 years, compared with only 7.6% in 1996.1 In 2006, the average age of women seeking infertility treatment was 35.6 years, and 16% of all women accessing in-vitro fertilisation (IVF) were aged over 40 years.2 However, all infertility treatments, including IVF, tend to work less well in women aged over 40 years (Box 2), and particularly poorly in women aged over 42 years.2 Therefore, one in six IVF patients now has an age factor complicating and reducing the success rate of the procedure.
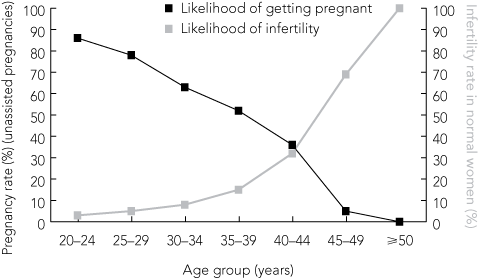
Pregnancy rates decrease exponentially after a maternal age of 37 years and enter an accelerated decline from 40 to 42 years.3 This age-related decline is due to poorer oocyte quality, probably associated with genetic factors and DNA fragmentation or deterioration.
Successful oocyte cryopreservation is a relatively recent development in assisted reproduction. Patients undergo a standard IVF cycle with ovarian hyperstimulation and surgical oocyte retrieval. The oocytes are then cryopreserved without fertilisation. After thawing, they are inseminated using intracytoplasmic sperm injection (ICSI). Successful outcomes have increased in the past 7 years, from occasional pregnancies to pregnancy rates in excess of 20% per thaw cycle, particularly in Europe, where restrictive embryo freezing laws4 have provided an impetus to focus on improving oocyte cryopreservation technology.
We have previously reported pregnancies from frozen/thawed oocytes in Australian women.5 These women were already undergoing IVF treatment and either had religious objections to embryo freezing or had problems obtaining sperm from their partners on the day of IVF. Other medical reasons to freeze oocytes include the need for a woman to undergo cytotoxic cancer treatment and the assessment that a woman is at high risk of premature menopause.
Our Queensland Fertility Group has produced Australia’s largest case series so far: nine pregnancies from frozen/thawed oocytes. Five women have delivered six healthy children (including a set of twins), there was one ectopic pregnancy, and three pregnancies are ongoing beyond 34 weeks. In two of these pregnancies, the oocytes were frozen to mitigate the risk of age-related fertility decline (Box 3). As far as we are aware, this is the first report of pregnancies in Australia using oocytes frozen for social rather than medical reasons.
It is difficult to assess the efficacy of frozen/thawed oocytes.6 The technology is new, and many units have only small pregnancy numbers. Although oocyte freezing is becoming more common, thaw cycles are rare. Pregnancy rates are dependent on the woman’s age at oocyte harvesting and freezing and on the technical expertise of the freeze/thaw process.
Experienced IVF units are achieving success rates with oocyte cryopreservation that approach those for fresh oocytes and frozen embryos.7-10 Survival rates of cryopreserved oocytes are 65%–80%, similar to those of frozen embryos, and fertilisation rates after insemination are 60%–80%, similar to those of fresh oocytes. Current data suggest there is one clinical pregnancy arising from every 17–25 thawed, surviving and inseminated oocytes,8 which is comparable to the figure of one pregnancy for every 15–20 usable, inseminated oocytes in a fresh IVF program.
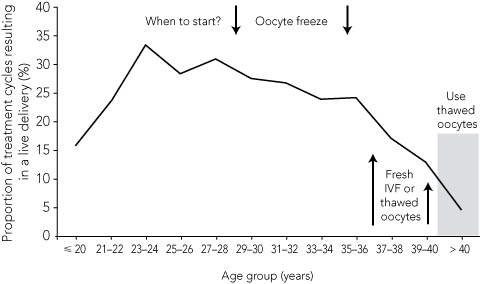
The ideal age for women to consider oocyte freezing is 31–35 years. Women in their late 20s still have time to find a partner or to have their oocytes frozen after the age of 30 years with little age penalty. However, many requests for oocyte freezing come from women aged 36–40 years, as illustrated by the two case studies described in Box 3. Although oocytes from patients in this age group will produce fewer pregnancies than those from women under 35 years, these patients may still benefit from pre-emptive oocyte freezing, given that the age-related decline in fertility after 42 years is so severe. An oocyte collected and frozen from a woman at 38 years may still work better than a fresh one collected at 43 years. Little benefit would be derived from freezing oocytes collected after the age of 40–41 years. The maximal mathematical benefit of cryopreservation for reproductive insurance is derived from oocytes harvested and frozen when a woman is under 35 years and used after the age of 40 years. Ideally, for health and social reasons, pregnancies should not be initiated in women older than 55 years.
Oocyte cryopreservation is an emerging and improving technology which, when used for social reasons, does not yet have universal support.11 Freezing methods are improving. The potential for using vitrification (ultra-rapid freezing that reduces the risk of damage to the oocyte) rather than standard slow-freeze technology is still to be realised, although the use of vitrification is becoming more common.12,13 If oocyte freezing for social reasons is likened to insurance, it is an expensive policy ($7000–$10 000 per cycle, and $300 per year to store the eggs) that may never be used if the woman becomes pregnant spontaneously or decides never to conceive. IVF is a stressful procedure that has uncommon but potentially serious risks associated with it, including ovarian hyperstimulation syndrome and the surgical risks of oocyte retrieval (such as infection or bleeding).
1 Pregnancy rates (unassisted pregnancies) and infertility rates in normal women, by age group, Australia and New Zealand*
 |
2 Proportion of non-donor fresh ART treatment cycles with oocyte retrieval that resulted in a live delivery, by maternal age group, Australia and New Zealand, 20042*†
 |
- David Molloy1
- Barbara A Hall2
- Marianne Ilbery3
- Jacqui Irving4
- Keith L Harrison5
- Queensland Fertility Group, Brisbane, QLD.
None identified.
- 1. Laws P, Abeywardana S, Walker J, Sullivan EA. Australia’s mothers and babies 2005. Sydney: Australian Institute of Health and Welfare National Perinatal Statistics Unit, 2007. (Perinatal Statistics Series No. 20; AIHW Cat. No. PER 40.)
- 2. Wang YA, Dean J, Badgery-Parker T, Sullivan EA. Assisted reproduction technology in Australia and New Zealand 2006. Sydney: Australian Institute of Health and Welfare National Perinatal Statistics Unit, 2008. (Assisted Reproduction Technology Series No. 12; AIHW Cat. No. PER 43.)
- 3. Committee on Gynecologic Practice of American College of Obstetricians and Gynecologists; Practice Committee of American Society for Reproductive Medicine. Age-related fertility decline: a committee opinion. Fertil Steril 2008; 90 (5 Suppl): S154-S155.
- 4. Ragni G, Allegra A, Anserini P, et al. The 2004 Italian legislation regulating assisted reproduction technology: a multicentre survey on the results of IVF cycles. Hum Reprod 2005; 20: 2224-2228.
- 5. Harrison KL, Lane MT, Osborn JC, et al. Oocyte cryopreservation as an adjunct to the assisted reproductive technologies [letter]. Med J Aust 2007; 186: 379. <MJA full text>
- 6. Edgar DH, Gook DA. How should the clinical efficiency of oocyte cryopreservation be measured? Reprod Biomed Online 2007; 14: 430-435.
- 7. Borini A, Cattoli M, Bulletti C, Coticchio G. Clinical efficiency of oocyte and embryo cryopreservation. Ann N Y Acad Sci 2008; 1127: 49-58.
- 8. Parmegiani L, Cognigni GE, Bernardi S, et al. Freezing within 2 h from oocyte retrieval increases the efficiency of human oocyte cryopreservation when using a slow freezing/rapid thawing protocol with high sucrose concentration. Hum Reprod 2008; 23: 1771-1777.
- 9. Shapiro BS, Daneshmand ST, Garner FC, et al. High ongoing pregnancy rates after deferred transfer through bipronuclear oocyte cryopreservation and post-thaw extended culture. Fertil Steril 2008; Nov 10. [Epub ahead of print.]
- 10. Chen SU, Lien YR, Chen HF, et al. Observational clinical follow-up of oocyte cryopreservation using a slow freezing method with 1,2-propanediol plus sucrose followed by ICSI. Hum Reprod 2005; 20: 1975-1980.
- 11. Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril 2008; 90 (5 Suppl): S134-S135.
- 12. Sher G, Keskintepe L, Mukaida T, et al. Selective vitrification of euploid oocytes markedly improves survival, fertilization and pregnancy-generating potential. Reprod Biomed Online 2008; 17: 524-529.
- 13. Lucena E, Bernal DP, Lucena C, et al. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 2006; 85: 108-111.





Abstract
Cryopreservation of unfertilised oocytes for later use in initiating pregnancy is now a viable technology, with acceptable pregnancy rates (over 20% per thaw cycle).
Oocyte cryopreservation used as a form of insurance against “social” (age-related) infertility can improve the lifetime chance of pregnancy in women who defer pregnancy into their late 30s or early 40s.
We report two pregnancies using oocytes that were frozen for social rather than medical reasons, as part of a larger series of nine pregnancies using cryopreserved oocytes.
Use of oocytes harvested and frozen from women aged under 35 years may more than double the chance of pregnancy for a 41-year-old woman.
The disadvantages of oocyte freezing for social infertility reasons include cost, the usual risks associated with in-vitro fertilisation, and the lack of a guarantee of eventual pregnancy.