Haemopoietic stem cell transplantation (HSCT) is an established procedure for treating children with a range of malignant and non-malignant diseases. The basic principle of HSCT involves a process of haematological and immunological reconstitution from infused haemopoietic stem cells (HSCs) following a course of bone marrow conditioning therapy. These cells can be the patient’s own HSCs (autologous) or cells from a donor (allogeneic). Conditioning regimens use combinations of chemotherapy and/or irradiation to suppress the recipient’s immune system and permit engraftment of the donor HSCs. Full-intensity conditioning is both myeloablative and immunosuppressive and serves to reduce the cancer burden directly and permit donor engraftment. Reduced-intensity conditioning regimens are non-myeloablative, but still induce sufficient immunosuppression to permit engraftment. They rely on the graft-versus-leukaemia effect of the donor’s immune system to produce the anti-cancer effect. Reduced-intensity HSCT is most often used when patients have been heavily pretreated for malignant disease or have comorbidities that preclude fully myeloablative therapy.1,2
The Australasian Bone Marrow Transplant Recipient Registry (ABMTRR) has collected data on HSCT in Australia since 1992 and New Zealand since 1998.3 By 2004, the ABMTRR had accrued more than 12 000 transplant records from 44 participating centres. Ownership of ABMTRR data is shared by the contributing centres, which are collectively represented by the Bone Marrow Transplant Society of Australia and New Zealand.
The ABMTRR aims to capture all cases of HSCT in Australasia each year so that the data can be used for administrative and research purposes. The list of corresponding hospitals is regularly assessed for completeness by correspondence with leading transplant physicians. At the beginning of each calendar year, all of the hospitals are contacted to ensure they have supplied all registrations from the previous year. In the year 2001, an estimated 99.5% of all HSCT activity in the two countries was captured by the Registry.3
Transplant-related mortality (TRM) refers to deaths that occur as a direct consequence of the transplant procedure, such as organ toxicity from conditioning therapy, or complications such as infection in the setting of immunosuppression from treatment for GVHD. TRM, defined here as deaths from transplant-related causes within the first 100 days and within the first year after transplantation, was calculated by the method of cumulative incidence, considering relapse, persistence, or progression of the original disease as competing risks.4,5
An alternative donor is a non-HLA-matched sibling, other family member or unrelated donor.
Over the period 1998–2006, 1259 paediatric HSCT procedures were performed, 522 autologous and 737 allogeneic (Box 1). Over this period, there was a decline in the number of autologous procedures performed, from 69 in 1999 and 2001 to 38 in 2006. The proportion of allogeneic procedures using grafts from alternative donors remained relatively constant at about 60%.
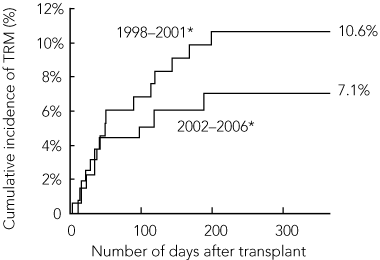
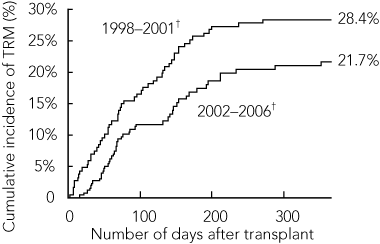
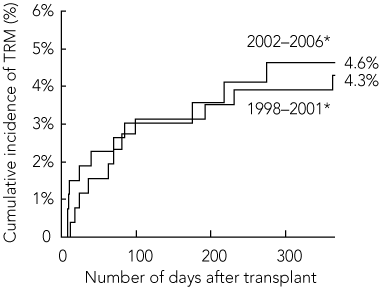
There was a non-significant reduction in TRM for allogeneic HSCT procedures between 1998–2001 and 2002–2006. For children undergoing HSCT from HLA-identical sibling donors, the cumulative incidence of TRM at 100 days after transplant was 6.8% in 1998–2001 compared with 5.1% in 2002–2006, while the 1-year TRM was 10.6% in 1998–2001 compared with 7.1% in 2002–2006 (Box 2). For children who received allogeneic transplants from alternative donors, the 100-day cumulative incidence of TRM was 16.5% in 1998–2001 compared with 11.7% in 2002–2006, while the 1-year TRM was 28.4% in 1998–2001 compared with 21.7% in 2002–2006 (Box 3). For children who had undergone autologous HSCT procedures, the 100-day cumulative incidence of TRM remained constant at about 3% between 1998 and 2006, while the 1-year TRM was 4.3% in 1998–2001 compared with 4.6% in 2002–2006 (Box 4).
The primary causes of death after HSCT for all patients are detailed in Box 5. The leading cause of death was persistence or relapse of the underlying disease, accounting for 28% of deaths within 100 days and 54% of deaths within 1 year of transplant. Other major causes of death within the first 100 days after transplant were veno-occlusive disease of the liver, infection, acute respiratory distress syndrome, multiple organ failure and GVHD.
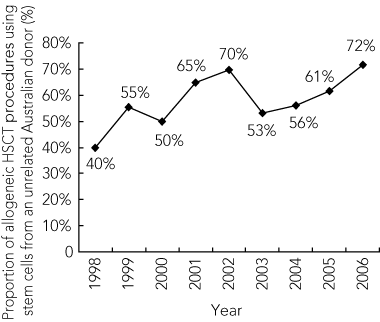
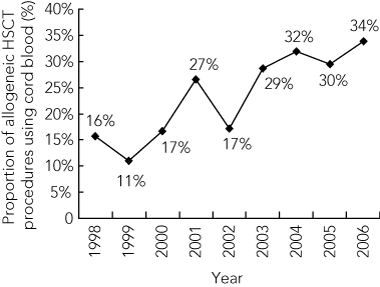
Since 1998, there has been a steady increase in the use of umbilical cord blood for paediatric HSCT in Australasia (Box 6). The increased use of cord blood units has also resulted in a modest increase in HLA-mismatched transplants, from 17% in 2000 to 26% in 2006.
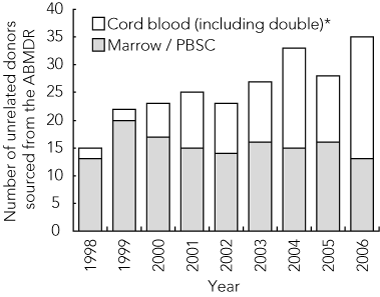
The growth of public banking of cord blood has led to a 32% increase in the number of children receiving unrelated HSCs from a donor sourced from the Australian Bone Marrow Donor Registry (Box 7). The number of bone marrow and peripheral blood stem cell donors sourced from the Australian Bone Marrow Donor Registry has remained relatively constant since 1998 (Box 8).
Characteristics of paediatric HSCT procedures conducted between 2002 and 2006 are detailed in Box 9. The median age of paediatric patients receiving a transplant during this period was 7 years (range, 0–19 years), with 61% of patients being male.
Allogeneic procedures accounted for 59% of HSCT activity. The most common indications for an allogeneic HSCT procedure were acute lymphoblastic leukaemia and acute myeloid leukaemia (Box 10). For allogeneic procedures (417 in total), the source of stem cells was marrow in 53%, peripheral blood stem cells in 17% and umbilical cord blood in 29%. Reduced-intensity (non-myeloablative) procedures accounted for only 6% of paediatric allogeneic HSCT procedures (Box 9).
Indications for autologous HSCT in Australian and New Zealand children from 2002 to 2006 are listed in Box 11. Autologous HSCT procedures were most commonly performed for solid tumours requiring very high-dose chemotherapy, such as neuroblastoma (23%), medulloblastoma (21%) and Ewing sarcoma (10%). Autologous HSCT for neuroblastoma was usually performed as a single myeloablative procedure, while HSCT for medulloblastoma and Ewing sarcoma/primitive neuroectodermal tumour (PNET) was most frequently given as staged reinfusions of stem cells following cycles of high-dose chemotherapy.
Analysis of ABMTRR data shows that, over the period 1998–2006, there was a fall in the number of autologous HSCT procedures performed. This is partly due to changes in treatment protocols for acute myeloid leukaemia, with autologous HSCT no longer part of standard treatment for this disease in children.6 Over the period 2002–2006, only 21 autologous HSCT procedures were performed for acute myeloid leukaemia, compared with 99 allogeneic procedures.
Autologous HSCT has an important role in a range of childhood cancers, including neuroblastoma, medulloblastoma, Ewing sarcoma/PNET, Hodgkin lymphoma and non-Hodgkin lymphoma.1,2 In our study, the most common indications for autologous HSCT in 2002–2006 were neuroblastoma, medulloblastoma and Ewing sarcoma.
Allogeneic transplantation is most frequently offered to children with high-risk and relapsed leukaemias, myelodysplastic syndromes, aplastic anaemia, congenital bone marrow failure syndromes, thalassaemia major, sickle cell anaemia and various inborn errors of metabolism.1,2 In our study, the most common indications for allogeneic HSCT in 2002–2006 were acute lymphoblastic leukaemia and acute myeloid leukaemia.
Over the 9-year period, we observed an increase in the proportion of allogeneic procedures using umbilical cord blood and using stem cells from unrelated donors. Cord blood provides a valuable source of HSCs for patients without a suitable bone marrow donor match. Furthermore, cord blood transplants produce similar disease-free survival for children with leukaemia when compared with allele-matched bone marrow transplants.7 Using HSCs from appropriately matched unrelated local donors or cord blood units is less expensive than using bone marrow or peripheral blood stem cells from international donors and reduces the time delay to transplant.
While not statistically significant, there was an encouraging trend towards reduced TRM for allogeneic transplants over the 9 years from 1998 to 2006. TRM observed in our study was comparable to TRM reported in international studies.7 Local trends in the indications for HSCT and in the use of alternative donors (including cord blood) also reflect contemporary international practice.1,2
1 Number of paediatric HSCT procedures performed in Australia and New Zealand, by type, 1998–2006 (n = 1259)
2 One-year cumulative incidence of TRM in children receiving allogeneic HSCT from HLA-identical sibling donors, 1998–2006
3 One-year cumulative incidence of TRM in children receiving allogeneic HSCT from alternative donors,* 1998–2006
7 Proportion of allogeneic HSCT procedures using stem cells from an unrelated donor sourced from the ABMDR, 1998–2006
ABMDR = Australian Bone Marrow Donor Registry. HSCT = haemopoietic stem cell transplantation.
- Andrew S Moore1
- Peter J Shaw2,3
- Andrew R Hallahan1
- Tina L Carter4
- Tatjana Kilo2
- Ian Nivison-Smith5
- Tracey A O’Brien6
- Heather Tapp7
- Lochie Teague8
- Shaun R Wilson2
- Karin Tiedemann9
- 1 Oncology/Haematology Service, Royal Children’s Hospital, Brisbane, QLD.
- 2 Oncology Unit, The Children’s Hospital at Westmead, Sydney, NSW.
- 3 Discipline of Paediatrics and Child Health, University of Sydney, Sydney, NSW.
- 4 Bone Marrow Transplant Program, Department of Haematology/Oncology, Princess Margaret Hospital for Children, Perth, WA.
- 5 Australasian Bone Marrow Transplant Recipient Registry, Sydney, NSW.
- 6 Centre for Children’s Cancer and Blood Disorders, Sydney Children’s Hospital, Sydney, NSW.
- 7 Women’s and Children’s Hospital, Adelaide, SA.
- 8 Starship Children’s Health, Auckland, NZ.
- 9 Children’s Cancer Centre, Royal Children’s Hospital, Melbourne, VIC.
None identified.
- 1. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813-1826.
- 2. Bollard CM, Krance RA, Heslop HE. Hematopoietic stem cell transplantation in pediatric oncology. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- 3. Nivison-Smith I, Bradstock KF, Dodds AJ, et al. Haemopoietic stem cell transplantation in Australia and New Zealand, 1992–2001: progress report from the Australasian Bone Marrow Transplant Recipient Registry. Intern Med J 2005; 35: 18-27.
- 4. Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. New York: John Wiley, 1995.
- 5. Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006; 12: 954-964.
- 6. Oliansky DM, Rizzo JD, Aplan PD, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 2007; 13: 1-25.
- 7. Eapen M, Rubinstein P, Zhang M-J, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 2007; 369: 1947-1954.





Abstract
Objective: To document haemopoietic stem cell transplantation (HSCT) activity and trends among paediatric patients in Australia and New Zealand.
Design, setting and participants: A retrospective analysis of data reported to the Australasian Bone Marrow Transplant Recipient Registry by the seven paediatric HSCT institutions in Australia and New Zealand over the 9-year period 1998–2006, with particular focus on the most recent years (2002–2006).
Main outcome measures: Types of HSCT performed; transplant-related mortality (TRM); stem cell sources; indications for HSCT; causes of death after HSCT.
Results: Over the period 1998–2006, 522 autologous HSCT procedures (41%) and 737 allogeneic procedures (59%) were performed. About 60% of allogeneic transplants involved alternative donors (donors other than a human leukocyte antigen-matched sibling). The use of umbilical cord blood as a source of haemopoietic stem cells has doubled since 1998, with 34% of allogeneic transplants in 2006 using cord blood. Over the period 2002–2006, the median age of patients receiving transplants was 7 years (range, 0–19 years). The most common indications for allogeneic HSCT were acute lymphoblastic leukaemia (33%) and acute myeloid leukaemia (24%). The most common indications for autologous HSCT were neuroblastoma (23%), medulloblastoma (21%) and Ewing sarcoma (10%). TRM at 1 year after transplant was 22% for alternative donor transplants, 7% for matched-sibling transplants and 5% for autologous transplants. Relapse or persistence of a child’s underlying condition accounted for 54% of all deaths within 1 year after transplant.
Conclusions: HSCT is an important procedure for children with a range of life-threatening illnesses. Local trends in the indications for HSCT, donor selection and TRM reflect contemporary international practice.