Several publications have directly linked adverse patient outcomes, including increased morbidity, mortality and inpatient length of stay (LOS), with overcrowding or LOS in the emergency department (ED).1-3 Recent studies have highlighted that reducing the percentage of turnaround time outliers for pathology tests can reduce LOS in the ED,4 even though standard laboratory processes have been mature for a long time.5 Alternative approaches that use point-of-care (POC) testing in the ED can reduce pathology test turnaround times and LOS in the ED,6,7 but this is not always the case.8,9
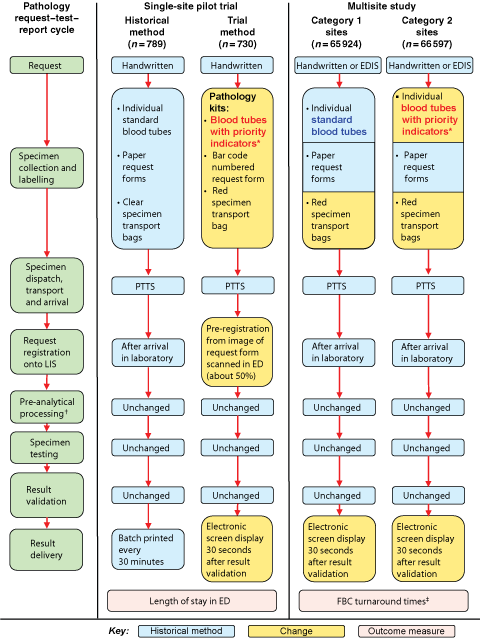
The pilot trial was a prospective observational analysis of de-identified trial data compared with historical control data. Historical data represented patients attending one hospital ED before implementation of a redesigned pathology process, and trial data represented patients attending the same ED after implementation of the redesigned pathology process (Box 1). Historical and trial data spanned October 2004 to March 2005, thus minimising potential seasonal effects on LOS in the ED, and included the triage category for each patient. The outcome measure was total ED time.10
The multisite study was a long-term retrospective analysis of de-identified data (with no exclusions) representing 132 521 FBC requests for patients attending seven EDs that implemented pathology process changes (coloured specimen transport bags alone, or coloured specimen bags plus blood tubes with a priority indicator) (Box 1). The outcome measures were the total pathology turnaround time (collected-to-validated time) and within-laboratory turnaround time (received-to-validated time) for FBC requests that did not require examination of a blood film and that were validated automatically via a computer algorithm.
COULTER Gen·S System 2 (Beckman Coulter, Fla, USA) and back-up COULTER MAXM;
COULTER Gen·S System 2; and
Two Sysmex XE-2100 (Sysmex, Kobe, Japan).
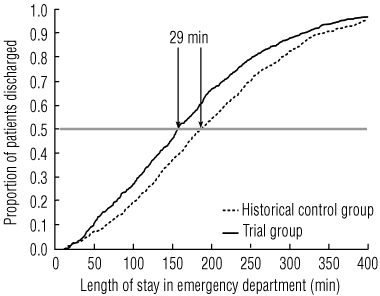
In the pilot trial, the proportion of patients in the trial group for each triage category was not statistically different to that of the control group (P = 0.08; Box 2). The redesigned pathology process was associated with a 29-minute (15.6%) reduction in median LOS in the ED (P < 0.001; Box 3). Considerable reductions in LOS were also revealed in triage categories 2, 3 and 4 (Box 4).
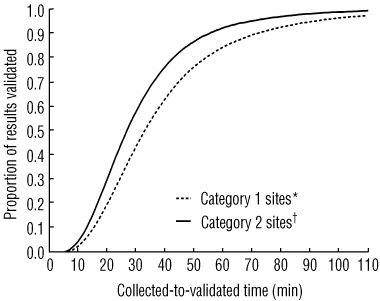
The use of coloured specimen transport bags plus blood tubes with an incorporated priority indicator showed a sustained and highly significant reduction in pathology test result turnaround time for FBC requests for patients attending EDs when compared with FBC requests that used coloured specimen bags alone (with standard blood tubes). A reduction in total pathology turnaround time was revealed for the entire patient population (P < 0.001; Box 5). The percentage improvements for the total laboratory turnaround time and within-laboratory turnaround time were similar (20.1% and 17.8% reductions, respectively; Box 6). Also, these proportional improvements are similar to the 15.6% proportional improvement in ED LOS in the pilot study.
In addition, there was a highly significant reduction in turnaround time outliers when the data were analysed according to timeframes specified in the Australian Council on Healthcare Standards (ACHS) Pathology Indicators (Box 7).12 Again, the percentage improvements for reduction in outliers for total laboratory turnaround times (49.7%) and within-laboratory turnaround times (44.2%) were similar.
The request–test–report cycle for pathology tests is a multistep process, involving up to 20 people for each sample. Accumulation of low rates of human error during the cycle can contribute to significant failure rates. Failure rates for urgent tests commonly requested by EDs can exceed 20%.13
Laboratories provide up to 80% of the information used by clinicians to make important medical decisions.14 However, in many instances, the percentage of laboratory turnaround time outliers has not changed significantly for up to 10 years.15-17 It has been suggested that timeliness of result reporting has not been a major focus in clinical laboratories16 and that, for at least some critical tests, the actual turnaround times fail to meet the expectations of the test provider and test user.16-19
Faster access to pathology results can reduce LOS in EDs and thereby improve clinical care and reduce total cost of care for individual patients.4,7,14,17,20,21
Several approaches have been used to try to improve laboratory services for EDs. Point-of-care testing, phlebotomists dedicated to ED collections, a dedicated satellite laboratory in the ED, and large-scale laboratory automation systems are costly and unlikely to meet the needs of most hospitals and laboratories as cost-effective solutions to poor turnaround times for analysis of samples from the ED.22,23
Pre- and post-analytical aspects of the pathology request–test–report cycle comprise a larger component of the testing process than the analysis. Improvements in laboratory turnaround time parameters have been achieved by the implementation of lean processing initiatives in the pre-analytical aspect of testing.5 These changes may be easier to achieve and more cost-effective than changes to specific analytical aspects of specimen analysis.5,17,18
By extrapolating our data to public EDs in Queensland, about 247 000 ED patients per year have an FBC that is autovalidated. Thus, the use of coloured specimen bags plus blood tubes with a priority indicator, compared with coloured specimen bags alone, would result in a time saving of more than 32 000 hours per year (which would benefit all ED patients), and more than 20 000 additional samples would meet the ACHS Pathology Indicator FBC collected-to-validated time of less than 60 minutes.12
There is an imperative to improve many aspects of the health care system,24 including patient flow in EDs, throughout the world. Rapid access to diagnostic tests is a prerequisite for good clinical outcomes.25 The initiatives described here are simple and cost-effective, and can be readily implemented at any hospital with an on-site laboratory.
2 Triage categories of patients attending an emergency department during a pilot trial of pathology process redesign
* Number of days for which data were collected and analysed. † According to the Australasian Triage Scale.11 |
|||||||||||||||
3 Proportion of patients discharged from the emergency department, by length of stay, during a pilot trial of pathology process redesign*
 | |||||||||||||||
4 Patient length of stay (LOS) in the emergency department during a pilot trial of pathology process redesign
5 Proportion of full blood count results validated, by collected-to-validated time, in a multisite study of pathology process redesign
 | |||||||||||||||
|
* Coloured specimen bags alone. † Coloured specimen bags plus blood tubes with a priority indicator. | |||||||||||||||
6 Mean laboratory turnaround times for full blood count requests from emergency departments in a multisite study of pathology process redesign
Category 2 sites: coloured specimen bags plus blood tubes with a priority indicator |
|||||||||||||||
7 Turnaround time outliers for full blood count (FBC) requests* from emergency departments in a multisite study of pathology process redesign
Category 2 sites: coloured specimen bags plus blood tubes with a priority indicator |
|||||||||||||||
* FBC requests not processed within standard timeframes according to the Australian Council on Healthcare Standards Pathology Indicators, version 3.12 † Received-to-validated time. ‡ Collected-to-validated time. |
|||||||||||||||
Received 10 November 2008, accepted 16 March 2009
- Andrew J Francis1,2,3
- Michael J Ray1
- Mary C Marshall1
- 1 The Prince Charles Hospital Laboratory Group, Pathology Queensland, Brisbane, QLD.
- 2 Priority Laboratory Services Australia, Brisbane, QLD.
- 3 School of Medicine, University of Queensland, Brisbane, QLD.
We thank The Prince Charles Hospital, the Innovation Branch of Queensland Health, the staff and volunteers of Queensland Health, and Harry Bartlett (Statistician, Queensland University of Technology) for their assistance and support.
Andrew Francis is a Director and indirect beneficial owner of companies that own the intellectual property rights associated with the specimen containers with an incorporated priority indicator that were used in this study. He may benefit from use or commercialisation of this intellectual property. He has received funding from Change Champions and the Australasian College for Emergency Medicine to attend conferences.
Funding for original trial work at The Prince Charles Hospital (January 2005 to March 2005) was jointly provided by Queensland Health and Priority Laboratory Services Australia. Funding for the multisite implementation was provided by the Innovation Branch of Queensland Health. These funding sources had no role in designing the study, collecting, analysing and interpreting the data, or preparing this article for publication.
Some of this work has been published in PathWay and The Australian newspaper and presented at conferences since 2005. Some is available at <http://www.changechampions.com.au/>.
- 1. Sprivulis PC, Da Silva JA, Jacobs IG, et al. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med J Aust 2006; 184: 208-212. <eMJA full text>
- 2. Cameron PA. Hospital overcrowding: a threat to patient safety? Med J Aust 2006; 184: 203-204. <eMJA full text>
- 3. Richardson DB. Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust 2006; 184: 213-216. <eMJA full text>
- 4. Holland LL, Smith LL, Blick KE. Reducing laboratory turnaround time outliers can reduce emergency department patient length of stay: an 11-hospital study. Am J Clin Pathol 2005; 124: 672-674.
- 5. Persoon TJ, Zaleski S, Frerichs J. Improving preanalytic processes using the principles of lean production (Toyota Production System). Am J Clin Pathol 2006; 125: 16-25.
- 6. Lee-Lewandrowski E, Corboy D, Lewandrowski K, et al. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center. Impact on test turnaround time and patient emergency department length of stay. Arch Pathol Lab Med 2003; 127: 456-460.
- 7. Blick KE. Economics of point-of-care (POC) testing for cardiac markers and B-natriuretic peptide (BNP). Point Care 2005; 4: 11-14.
- 8. Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. BMJ 1998; 316: 1052-1057.
- 9. Plerhoples W, Zwemer FL Jr, Bazarian J. Point of care pregnancy testing provides staff satisfaction but does not change ED length of stay. Am J Emerg Med 2004; 22: 460-464.
- 10. Australasian College for Emergency Medicine. Standard terminology. Emerg Med (Fremantle) 2002; 14: 337-340.
- 11. Australasian College for Emergency Medicine. The Australasian Triage Scale. Emerg Med (Fremantle) 2002; 14: 335-336.
- 12. Australian Council on Healthcare Standards. Clinical indicators users’ manual 2008 — pathology indicators. Version 3. Sydney: ACHS, 2007: 640-659.
- 13. Australian Council on Healthcare Standards. Australasian clinical indicator report 1998–2006. 8th ed. Sydney: ACHS, 2007: 474-496.
- 14. Browning RA. The labor shortage, patient safety, and length of stay: new era of change agents prompts process improvements through lab automation. JALA Charlottesv Va 2004; 9: 24-27.
- 15. Australian Council on Healthcare Standards. Australasian clinical indicator report 1998 — 2005. 7th ed. Sydney: ACHS, 2006: 397-416.
- 16. Howanitz JH, Howanitz PJ. Laboratory results. Timeliness as a quality attribute and strategy. Am J Clin Pathol 2001; 116: 311-315.
- 17. Steindel SJ, Howanitz PJ. Physician satisfaction and emergency department laboratory test turnaround time. Arch Pathol Lab Med 2001; 125: 863-871.
- 18. Valenstein P. Laboratory turnaround time. Am J Clin Pathol 1996; 105: 676-688.
- 19. Novis DA, Jones BA, Dale JC, et al. Biochemical markers of myocardial injury test turnaround time: a College of American Pathologists Q-Probes study of 7020 troponin and 4368 creatine kinase-MB determinations in 159 institutions. Arch Pathol Lab Med 2004; 128: 158-164.
- 20. Leman P, Guthrie D, Simpson R, et al. Improving access to diagnostics: an evaluation of a satellite laboratory service in the emergency department. Emerg Med J 2004; 21: 452-456.
- 21. Holland LL, Smith LL, Blick KE. Total laboratory automation can help eliminate the laboratory as a factor in emergency department length of stay. Am J Clin Pathol 2006; 125: 765-770.
- 22. Sheppard C, Franks N, Nolte F, et al. Improving quality of patient care in an emergency department: a laboratory perspective. Am J Clin Pathol 2008; 130: 573-577.
- 23. Fermann GJ, Suyama J. Point of care testing in the emergency department. J Emerg Med 2002; 22: 393-404.
- 24. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press, 2001: 39-60. http://www.nap.edu/openbook/0309072808/html/R1.html (accessed Nov 2008).
- 25. The Australian Medical Association. Position statement on quality and safety in public hospitals. Canberra: AMA, 2006: 5. http://www.ama.com.au/web.nsf/doc/WEEN-6W83XC (accessed Nov 2008).





Abstract
Objectives: To determine whether redesign of pathology processes, including indicators of sample priority, could reduce patient length of stay (LOS) in an emergency department (ED), and assess the long-term impact of two indicators of sample priority on pathology clinical performance indicators for ED samples.
Design, setting and participants: Two observational studies of de-identified data from standard databases were conducted — a single-site pilot trial of patients attending the ED of one hospital compared with historical controls, and a multisite study of 132 521 full blood count (FBC) requests for patients attending seven EDs that utilised either of two pathology process changes (coloured specimen transport bags alone, or coloured specimen bags plus blood tubes with a priority indicator).
Main outcome measures: LOS in the ED was measured for the pilot trial, and collected-to-validated times for FBCs that fulfilled computer algorithm validation rules were measured for the multisite study.
Results: In the pilot trial, the redesigned pathology process resulted in a 29-minute reduction (15.6%) in the median ED LOS for all patients (P < 0.001) compared with historical controls. In the multisite study, use of coloured specimen bags plus blood tubes with a priority indicator resulted in an 8-minute reduction (20.1%) in mean collected-to-validated times for FBC requests compared with FBC requests that used coloured specimen bags alone (P < 0.001).
Conclusions: Our pilot trial revealed a direct relationship between pathology process design and LOS in the ED, suggesting that redesigned pathology processes can significantly reduce LOS in the ED. Our multisite study showed that collecting samples directly into blood tubes with an incorporated priority indicator reduces pathology test turnaround times. These data suggest that LOS in the ED can be significantly reduced by simple changes to pathology processes, such as collecting samples directly into specimen containers with an incorporated priority indicator.