Care in a stroke unit improves long-term outcomes for stroke patients, underpinning the enhanced organisation of stroke services now underway across Australia.1,2 An important component of organised acute stroke care is thrombolytic therapy with intravenous tissue plasminogen activator (tPA),2,3 which was licensed for use in Australia in 2003. This highly cost-effective therapy4-6 substantially reduces major disability when used in an expert setting.7,8
Only a small proportion of stroke patients currently receive thrombolytic therapy.9-11 A major factor limiting patient access is that few arrive at expert stroke centres within the narrow 3-hour treatment window. Patient access to tPA could potentially be improved by extending organised stroke care through a cooperative system that crosses the traditional clinical and administrative boundaries of the acute stroke unit, emergency department (ED) and pre-hospital sector.
Where extended organised acute care has been successfully implemented, up to 20% of patients with ischaemic stroke are treated with tPA.12-14 Collaboration with ambulance services is recognised as a key to decreasing the time to presentation for patients with acute stroke. Pre-hospital stroke screening tools, such as the Los Angeles Prehospital Stroke Screen (LAPSS)15 and the Face Arm Speech Test (FAST),16 aid this process. These tools have acceptable diagnostic accuracy in the field and are important components of protocols for pre-hospital stroke care.
lack of awareness among ambulance officers of the evidence and requirements for rapid access to organised acute stroke care;
delivery of potentially thrombolysis-eligible patients to hospitals within the Hunter Region not equipped to provide a stroke thrombolysis service; and
delays in acute stroke team notification on arrival of potentially thrombolysis-eligible stroke patients in the ED.
The pre-hospital stroke assessment tool was adapted from the United Kingdom FAST tool,16 to complement the National Stroke Foundation FAST community awareness campaign and existing assessment tools used by NSW ambulance officers. The local GAS-T tool has four elements:
G = glucose in the “normal” range (4–17 mmol/L);
A = arm drift, grip strength;
S = speech disturbance; and
T = time since symptom onset (Box 1).
An important difference from the UK tool is the replacement of the more difficult facial asymmetry test, “F”, with a simple finger-prick glucose test, “G”. Hyperglycaemia is a relative contraindication to tPA, being associated with poor outcomes and higher rates of haemorrhagic transformation,17 while hypoglycaemia is a known stroke mimic and may have led to reduced diagnostic accuracy in the field. “T” was added to the tool to emphasise the importance of the 2-hour time window. Although tPA is licensed for treatment of patients with ischaemic stroke within 3 hours of symptom onset, the third hour was allocated to in-hospital pretreatment procedures, such as patient assessment, imaging and gaining patient assent.
Tertiary outcome measures were based on clinical outcome at 3 months measured by the modified Rankin score (a validated disability measure where 0–1 = excellent outcome; 2 = functional independence; 3 =moderate disability; and 4–5 = major disability).18,19 Outcomes were compared between patients treated with tPA in the PAST group, and patients in the NINDS (National Institute of Neurological Disorders and Stroke) tPA trial7 and the SITS (Safe Implementation of Thrombolysis in Stroke) tPA registry.20
During the 6-month PAST protocol period, 232 patients presented to the John Hunter Hospital ED with an initial diagnosis of stroke, and 140 of these (60%) had a final discharge diagnosis of ischaemic stroke (Box 2). Of the 232 patients, 122 (53%) had a defined onset time within the previous 24 hours, and 51 of the 232 were delivered to John Hunter Hospital using the PAST protocol.
In the 6-month control period, 205 patients presented to the John Hunter Hospital ED with an initial diagnosis of stroke, and 107 (52%) had a final discharge diagnosis of ischaemic stroke (Box 2). Of the 205 patients, 61 (30%) had a defined onset time within the previous 24 hours.
There was a statistically significant increase in the proportion of ischaemic stroke patients who received intravenous tPA therapy in the PAST group compared with the control group: 21.4% (95% CI, 14.6%–28.2%) versus 4.7% (95% CI, 1.5%–10.6%); P < 0.001) (Box 3). Expressed as a percentage of all patients presenting to the ED with an initial diagnosis of stroke, 12.9% (95% CI, 8.6%–17.2%) received tPA during the PAST period compared with 2.4% (95% CI, 0.8%–5.6%) during the control period (P < 0.001).
Process of care measures are shown in Box 4. The median time from symptom onset to hospital arrival was reduced by 1 hour in the PAST group compared with the control group (90.5 v 150 min; P = 0.004). The median ED transit time was also reduced in the PAST group (232.5 v 361 minutes in the control group; P < 0.001). Door to needle times among those who received tPA were similar in the two groups (91.5 v 89 minutes; P = 0.40), but very few patients in the control group (five) received tPA.
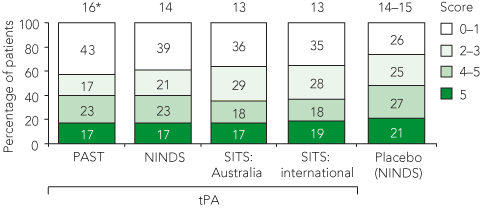
Three-month outcomes for patients treated with tPA during the PAST period compared favourably with those for tPA-treated patients in the benchmark NINDS tPA trial,7 the SITS international phase IV tPA registry,20 and published Australian audits of tPA implementation8,21 (Box 5). Baseline stroke severity measured by the National Institutes of Health Stroke Scale (NIHSS)7 was worse in our cohort than comparators. Despite this, 43% of our tPA group had minimal or no disability at 3 months, which is at least equivalent to national and international benchmarks.19,22
Audits of tPA use in Australia currently indicate that only 0.9% of all ischaemic stroke patients receive this treatment.9 Given the international evidence that changes in the process of care can substantially increase tPA use,2,23,24 and given the cost effectiveness of tPA therapy,4 there is a clear need to develop support systems and care models to help stroke clinicians in Australia redesign pre-hospital and emergency care systems for acute stroke. Published pre-hospital stroke care protocols have tended to focus on single components of the process of care, such as assessment,25 ED triage,26,27 or in-hospital fast-track protocols.28 PAST is the first Australian stroke protocol that covers both pre-hospital assessment and ED care.
We found that the PAST protocol reduced pre-hospital and ED delays to treatment and significantly increased the proportion of patients treated with intravenous tPA. This partly reflects the substantial reduction in time from symptom onset to hospital arrival during the implementation period. The proportion of patients who arrived at our centre within 2 hours of symptom onset was similar to the proportion who arrived within 3 hours of symptom onset at another Australian centre that had no pre-hospital stroke ambulance protocol in place.23 An additional major benefit of the PAST protocol was the substantial reduction in ED transit time, probably reflecting earlier mobilisation of the stroke team, allowing advance discussions with hospital bed managers.
Our study had potential limitations, particularly the use of historical controls (albeit prospectively collected) and the non-randomised design. This may have resulted in a higher proportion of more severe strokes in the PAST group (Box 2), which may have contributed to the higher rate of early presentation in the PAST period, as these particular strokes may be more readily diagnosed by ambulance officers in the field. Additionally, the retrieval of patients from outside the usual hospital catchment area may have increased the proportion of patients receiving thrombolysis. However, this was a specific aim of the PAST protocol. Indeed, as in most Australian regional areas, these patients would not have had access to thrombolytic therapy before implementation of PAST. While the PAST protocol worked well in the Hunter Region, its geography and systems differ from those in larger metropolitan areas of Australia, where implementation of a similar protocol may be more challenging.
Most importantly, the substantial increase in the proportion of patients treated with tPA during the PAST period is likely to have an impact on stroke-related disability in our region. Using a number needed to treat (NNT) of eight to prevent one case of major disability,7 and with an absolute increase of 25 patients treated during the PAST period, we estimate that over the 6-month period, the protocol prevented three patients from requiring nursing home care. Not only is this meaningful at an individual patient level, but the impact of the protocol being implemented throughout all Australian metropolitan and large regional areas could be substantial. For example, assuming implementation of PAST resulted in a modest increase of 5% in patients treated with thrombolysis Australia-wide (compared with the 16% increase seen in our study), then about 2500 more patients would be treated per annum. Using the same NNT, 312 patients would be saved from major disability, with a cost saving of $31.2 million (from reduced need for rehabilitation and nursing home care) in the first 12 months.4
The prioritisation and preferential transport by ambulance services of patients with suspected stroke to centres with stroke units conforms with the recommendations of the National Strategic Improvement Framework for Heart, Stroke and Vascular Disease28 and the 2007 National Stroke Foundation guidelines.2 While a significant commitment on the part of health care providers would be needed, implementation of “fast-track” protocols such as PAST, supported by well organised, hospital-based stroke care nationwide, would have a substantial impact on stroke-related disability, as well as the resultant burdens and costs to the individual and community. Relatively few therapies have an absolute cost saving. When highly effective therapies are available for a disease of such enormous community impact as stroke, directing efforts and resources towards redesign of pre-hospital and acute stroke care systems appears both desirable and justified.
2 Patient characteristics, by treatment group
Onset within previous 24 h (% of patients with data available) |
|||||||||||||||
Presentation within 2 h of onset (% of patients with data available) |
|||||||||||||||
3 Number of patients with ischaemic stroke and number who received tPA, by treatment group
tPA = tissue plasminogen activator. PAST = Pre-hospital Acute Stroke Triage. |
|||||||||||||||
4 Process measures for patients with a defined symptom onset time within 24 hours of presentation to hospital and for patients who received tPA (values are median and IQR)
5 Distribution of patient outcomes at 3 months after stroke, based on modified Rankin scores, in our study (PAST protocol) and other Australian and international studies
 | |||||||||||||||
|
NINDS = National Institute of Neurological Disorders and Stroke tPA trial.7 SITS = Safe Implementation of Thrombolysis in Stroke tPA registry.20 * Values above columns are the median baseline NIHSS (National Institutes of Health Stroke Scale) scores for each cohort. | |||||||||||||||
Received 9 January 2008, accepted 19 June 2008
- Debbie A Quain1,2
- Mark W Parsons1,2,3
- Allan R Loudfoot4
- Neil J Spratt1,3
- Malcolm K Evans1
- Michelle L Russell1
- Angela T Royan1
- Andrea G Moore1
- Ferdinand Miteff1,2
- Carolyn J Hullick2
- John Attia3,1
- Patrick McElduff1,3
- Christopher R Levi1,2
- 1 Hunter Medical Research Institute, Newcastle, NSW.
- 2 John Hunter Hospital, Newcastle, NSW.
- 3 University of Newcastle, Newcastle, NSW.
- 4 Northern Ambulance Service of NSW, Newcastle, NSW.
Christopher Levi, Mark Parsons and Neil Spratt have received speaker fees and educational grants from Boehringer Ingelheim.
- 1. Cadilhac DA, Lalor EE, Pearce DC, et al. Access to stroke care units in Australian public hospitals: facts and temporal progress. Intern Med J 2006; 36: 700-704.
- 2. Crimmins D, Bladin C, Lalor E. Clinical guidelines for acute stroke management 2007. Melbourne: National Stroke Foundation, 2007.
- 3. Hack W, Kaste M, Bogousslavsky J, et al. European Stroke Initiative Recommendations for Stroke Management — update 2003. Cerebrovasc Dis 2003; 16: 311-337.
- 4. Moodie ML, Carter R, Mihalopoulos C, et al. Trial application of a Model of Resource Utilization, Costs, and Outcomes for Stroke (MORUCOS) to assist priority setting in stroke. Stroke 2004; 35: 1041-1046.
- 5. Fagan SC, Morgenstern LB, Petitta A, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology 1998; 50: 883-890.
- 6. Sandercock P, Berge E, Dennis M, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke 2004; 35: 1490-1497.
- 7. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581-1587.
- 8. Bray JE, Coughlan K, Bladin C. Thrombolytic therapy for acute ischaemic stroke: successful implementation in an Australian tertiary hospital. Intern Med J 2006; 36: 483-488.
- 9. Randahawa D. National stroke audit organisational report acute services 2007. Melbourne: National Stroke Foundation, 2007: 6-8.
- 10. Qureshi AI, Fareed K, Nasar A, et al. Thrombolysis for ischemic stroke in the United States: data from national hospital discharge survey 1999–2001. Neurosurgery 2005; 57: 647-654.
- 11. McCormick M, Baird M, Bone I, Muir K. Implementation of stroke thrombolysis in the United Kingdom. Glasgow: University of Glasgow, 2006.
- 12. Grotta JC, Burgin WS, El-Mitwalli A, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol 2001; 58: 2009-2013.
- 13. Schmulling S, Grond M, Rudolf J, Heiss W. One-year follow-up in acute stroke patients treated with rtPA in clinical routine. Stroke 2000; 31: 1552-1554.
- 14. Wojner-Alexandrov AW, Rodriguez D, Persse D, et al. Houston paramedic and emergency stroke treatment and outcomes study (HoPSTO). Stroke 2005; 36: 1512-1518.
- 15. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke 2000; 31: 71-76.
- 16. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003; 34: 71-76.
- 17. Weir CJ, Murray GD, Dyker AG, et al. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long term follow up study. BMJ 1997; 314: 1303-1306.
- 18. Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke 1988; 19: 1497-1500.
- 19. Rankin J. Cerebral vascular accidents in patients over the age of 60. I. General considerations. Scott Med J 1957; 2: 127-136.
- 20. Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275-282.
- 21. Szoeke CEI, Parsons MW, Butcher KS, et al. Acute stroke thrombolysis with intravenous tissue plasminogen activator in an Australian tertiary hospital. Med J Aust 2003; 178: 324-328. <MJA full text>
- 22. Batmanian JJ, Lam M, Matthews C, et al. A protocol-driven model for the rapid initiation of stroke thrombolysis in the emergency department. Med J Aust 2007; 187: 567-570. <MJA full text>
- 23. Lindsberg PJ, Happola O, Kallela M, et al. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology 2006; 67: 334-336.
- 24. Bray JE, Martin J, Cooper G, et al. An interventional study to improve paramedic diagnosis of stroke. Prehosp Emerg Care 2005; 9: 297-302.
- 25. Morris DL, Rosamond W, Madden K, et al. Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey. Stroke 2000; 31: 2585-2590.
- 26. Rajajee V, Saver J. Prehospital care of the acute stroke patient. Tech Vasc Interv Radiol 2005; 8: 74-80.
- 27. Tilley BC, Lyden PD, Brott TG, et al. Total quality improvement method for reduction of delays between emergency department admission and treatment of acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Arch Neurol 1997; 54: 1466-1474.
- 28. National Health Priority Action Council. National Service Improvement Framework for Heart, Stroke, and Vascular Disease. Canberra: Australian Government Department of Health and Ageing, 2006: 115.






Abstract
Objective: To assess the effectiveness of the PAST (Pre-hospital Acute Stroke Triage) protocol in reducing pre-hospital and emergency department (ED) delays to patients receiving organised acute stroke care, thereby increasing access to thrombolytic therapy.
Design: Prospective cohort study using historical controls.
Setting: Hunter Region of New South Wales, September 2005 to March 2006 (pre-intervention) and September 2006 to March 2007 (post-intervention).
Participants: Consecutive patients presenting with acute stroke to a regional, tertiary referral hospital.
Intervention: PAST protocol, comprising a pre-hospital stroke assessment tool for ambulance officers, an ambulance protocol for hospital bypass for potentially thrombolysis-eligible patients, and pre-hospital notification of the acute stroke team.
Main outcome measures: Proportion of patients who received intravenous tissue plasminogen activator (tPA), process of care time points (symptom onset to ED arrival, ED arrival to tPA treatment, and ED transit time), and clinical outcomes of patients treated with tPA.
Results: The proportion of ischaemic stroke patients treated with tPA increased from 4.7% (pre-intervention) to 21.4% (post-intervention) (P < 0.001). Time point outcomes also improved, with a reduction in median times from symptom onset to ED arrival from 150 to 90.5 min (P = 0.004) and from ED arrival to stroke unit admission from 361 to 232.5 minutes (P < 0.001). Of those treated with tPA, 43% had minimal or no disability at 3 months.
Conclusions: Organised pre-hospital and ED acute stroke care increases patient access to tPA treatment, which is proven to reduce stroke-related disability.