Stroke is the third commonest cause of death and a major cause of disability in Australia.1 Intravenous therapy with tissue plasminogen activator (tPA) is currently the only approved medical therapy for patients with acute ischaemic stroke. Patients who receive tPA within 3 hours of ischaemic stroke onset are at least 30% more likely to have little or no disability compared with those who do not,2 with a number needed to treat to obtain a clinical benefit as low as three.3
Even in international centres, however, only a small proportion of patients (2%–10%) with ischaemic stroke receive thrombolytic therapy.4 In Australia, the proportion is lower; an audit of eight metropolitan tertiary referral hospitals from five Australian states found that < 1% of ischaemic stroke patients received thrombolytic therapy.5
The major reason for the small numbers receiving thrombolysis is delayed presentation of stroke patients to hospitals. However, even in stroke patients who present to hospital within the 3-hour timeframe, the use of thrombolysis has remained controversial, with questions about whether this treatment can be broadly and safely administered in the emergency department (ED).6 Hence, thrombolysis for ischaemic stroke has been limited to a few centres in Australia with a “stroke team”, usually comprising stroke neurologists, stroke fellows, registrars and nurses who assess and administer therapy.7
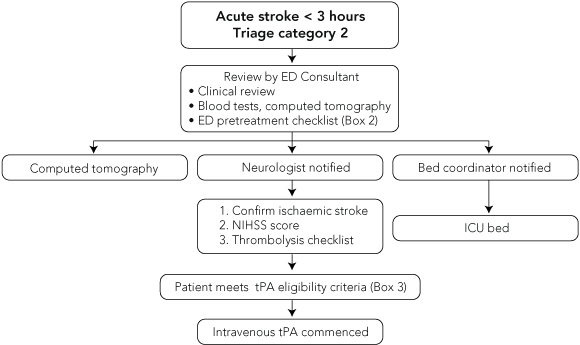
The acute stroke care protocol (Box 1) was initiated in the ED at St Vincent’s Hospital (SVH), Sydney, in October 2004, and extended to 24-hour, 7-days-a-week coverage in December 2004. The protocol was developed jointly by neurology, emergency and intensive care unit physicians and nurses. The working party provided education to staff in the ED, including triage nurses, radiology and acute stroke unit staff, before implementing the protocol and quarterly thereafter.
All patients admitted to the stroke unit at SVH are prospectively entered into the SVH stroke database, which records stroke onset and arrival times, demographics, and vascular risk factors, and is used to generate a discharge summary.8 In addition, stroke subtype according to the Oxfordshire Classification and stroke mechanism according to the modified TOAST criteria are recorded for each patient.9 Patients with ischaemic stroke receiving thrombolytic therapy were also entered into the Safe Implementation of Thrombolysis in Stroke (SITS) International Registry (http://www.acutestroke.org), an Internet-based, data-entry monitoring system designed for auditing the efficacy and safety of routine thrombolytic therapy in acute ischaemic stroke.
All patients presenting to the ED within 3 hours of stroke onset were triaged as category 2 and underwent rapid assessment (within 10 minutes) by a senior emergency physician (Box 1). Initial assessment included a brief history and examination focused on time of onset of symptoms and identifying major contraindications to thrombolysis. Monitoring of vital signs, intravenous cannulation, and investigations, including blood tests and urgent computed tomography (CT) brain scans, were initiated according to a standing order (Box 2).
The decision to treat with tPA was made by the attending neurologist after reviewing the patient and CT scan, documenting the severity of stroke according to the National Institutes of Health Stroke Scale (NIHSS), and completing a thrombolysis inclusion/exclusion criteria checklist (Box 3). The presence of early ischaemic changes on CT was not considered a contraindication. The patient and/or next of kin were informed about the risks and benefits of thrombolysis.
Baseline characteristics for the 15 patients receiving thrombolytic therapy are shown in Box 4. The mean age was 67 years, 53% were male, and the median baseline NIHSS score was 11 (range, 5–18). The mean systolic blood pressure was 144 mmHg, and mean diastolic blood pressure was 81 mmHg. The baseline stroke severity was similar to that in the SITS registry.10
Significant early improvement at 24 hours was observed in seven (47%; 95% CI, 21%–73%) of 15 stroke patients receiving thrombolysis. Independence at 3 months (mRS, 0–2) was seen in 67% (10/15; 95% CI, 38%–88%) of treated patients, compared with 54.8% (95% CI, 53.5%–56%) in the SITS registry data.10 Of this group, five improved completely (mRS = 0), two had no significant disability (mRS = 1) and three had slight disability (mRS = 2). Thus, seven of 15 patients (47%; 95% CI, 21%–73%) achieved an excellent functional outcome (mRS, 0–1) at 3 months.
In the 15 patients receiving thrombolysis, no ICH (0/15; 95% CI, 0–22%) was observed. One patient developed orolingual angio-oedema and was managed medically without an adverse outcome.11
In stroke centres with no experience of acute stroke thrombolysis, routine use of tPA can be implemented with the safety and efficacy demonstrated in randomised clinical trials, provided there is strict adherence to and monitoring of protocols.10 Our 24-hour acute stroke management protocol was successful in selecting patients eligible for thrombolysis. The proportion of overall stroke patients treated with thrombolysis (14% of all stroke admissions) and the use of thrombolysis in eligible patients (95%) compare favourably with results from other multicentre12 and single centre13 studies. Our small sample size leads to imprecise estimates, but stroke severity at study entry and clinical improvement at 24 hours and 3 months compare favourably with the SITS registry cohort and pooled results from meta-analysis.14 Importantly, our protocol identified patients with one or more contraindications15 for thrombolysis (21 of 35 patients). There were no significant protocol violations, which have been associated with a higher incidence of adverse outcomes, principally ICH.16 The absence of ICH in the treated patients is reassuring, and suggests that our protocol is safe, although a larger sample is needed to identify the true ICH rate. In the SITS registry, 1.7% of patients have symptomatic ICH.10
The major reason for the small proportion of stroke patients eligible for thrombolytic therapy is delayed presentation.17 During our study period, only 40% of patients with stroke presented within 3 hours of symptom onset. For patients presenting within the 3-hour time window, the most common reasons for exclusion from thrombolytic therapy were minor or rapidly resolving symptoms, and presence of haemorrhage on initial CT.
The median time from stroke onset to treatment of 155 minutes and the median door-to-needle time of 87 minutes are similar to times reported in a large series from Canada,16 but are longer than the “best practice guidelines” recommended by the National Institute of Neurological Disorders and Stroke (NINDS) study group.18 Our thrombolysis working party met 3-monthly to provide ongoing staff education, assess adherence to protocols and post-thrombolysis nursing standards of care, and monitor quality parameters and discuss quality improvements. This has resulted in a downward trend for door-to-needle times, although further efficiencies are required to meet best practice guidelines.
2 Emergency department (ED) pretreatment checklist for acute stroke patients presenting within 3 hours of onset
3 Thrombolysis inclusion and exclusion criteria checklist
- Julia J Batmanian1
- Meeyin Lam1
- Caitlin Matthews1
- Andrew Finckh1
- Martin Duffy1
- Robert Wright1
- Bruce J Brew2,1
- Romesh Markus1,2
- 1 St Vincent’s Hospital, Sydney, NSW.
- 2 University of New South Wales, Sydney, NSW.
The study was supported by the Ladies Committee Sr Mary Bernice Research Grant (St Vincent’s Clinic Foundation). The support of the staff of the emergency department, intensive care unit and acute stroke care unit is gratefully acknowledged. We would like to especially thank Julie Gawthorne and Megan Vidler for their contribution to coordinating and promoting the acute stroke protocol.
Bruce Brew has received reimbursement from GlaxoSmithKline, Boehringer Ingelheim, Gilead, and Biogen Idec for giving educational lectures to medical personnel. Romesh Markus has received honoraria and grants from Boehringer-Ingelheim.
- 1. Hankey GJ. Transient ischaemic attacks and stroke. Med J Aust 2000; 172: 394-400.
- 2. The NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med 1995; 333: 1581-1587.
- 3. Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol 2004; 61: 1066-1070.
- 4. Qureshi AI, Kirmani JF, Sayed MA, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology 2005; 64: 2115-2120.
- 5. Duffy BK, Phillips PA, Davis SM, et al. Evidence-based care and outcomes of acute stroke managed in hospital specialty units. Med J Aust 2003; 178: 318-323. <MJA full text>
- 6. Hoffman JR. Tissue plasminogen activator (tPA) for acute ischaemic stroke: why so much has been made of so little. Med J Aust 2003; 179: 333-334. <MJA full text>
- 7. Szoeke CE, Parsons MW, Butcher KS, et al. Acute stroke thrombolysis with intravenous tissue plasminogen activator in an Australian tertiary hospital. Med J Aust 2003; 178: 324-328. <MJA full text>
- 8. Faux S, Lam M, Fuller K, Markus R. Combined stroke database and electronic discharge summary: The St Vincent’s Hospital (Sydney) Prospective Stroke Registry [abstract]. Intern Med J 2005; 35: A15.
- 9. Adams H Jr, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35-41.
- 10. Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275-282.
- 11. Hill MD, Barber PA, Takahashi J, et al. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ 2000; 162: 1281-1284.
- 12. Katzan IL, Hammer MD, Hixson ED, et al. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 2004; 61: 346-350.
- 13. Koennecke HC, Nohr R, Leistner S, Marx P. Intravenous tPA for ischemic stroke team performance over time, safety, and efficacy in a single-center, 2-year experience. Stroke 2001; 32: 1074-1078.
- 14. The ATLANTIS ECASS and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768-774.
- 15. Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke 2005; 36: 916-923.
- 16. Hill MD, Buchan AM, for The Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005; 172: 1307-1312.
- 17. California Acute Stroke Pilot Registry Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology 2005; 64: 654-659.
- 18. Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA Stroke Study. Neurology 2000; 55: 1649-1655.





Abstract
Objective: To assess efficacy and safety of a 24-hour comprehensive protocol-driven model for rapid assessment and thrombolysis of stroke patients in the emergency department.
Design: Prospective open observational study.
Participants and setting: All patients with acute stroke presenting within 3 hours to the St Vincent’s Hospital (Sydney) emergency department between 1 December 2004 and 30 July 2005.
Main outcome measures: Proportion of patients treated, patient demographics, clinical outcome, adverse events and time to treatment parameters.
Results: 134 patients (100 stroke; 34 transient ischaemic attack) were admitted to the stroke unit during the study period. Of the 100 stroke patients, 40 presented within 3 hours of symptom onset. Fifteen patients had no contraindications and received intravenous thrombolysis. At 3 months, 10 patients (67%) were independent (modified Rankin score [mRS], 0–2) and seven (47%) had an excellent functional outcome (mRS ≤ 1). Symptomatic intracranial haemorrhage was not observed. The median time from symptom onset to tissue plasminogen activator treatment was 155 minutes (range, 105–197 min). Median onset-to-door, door-to-computed tomography, and door-to-needle times were 48, 25, and 87 minutes, respectively.
Conclusion: Rapid assessment of stroke in the emergency department according to a comprehensive protocol allows identification and treatment of acute ischaemic stroke patients eligible for thrombolysis.