In 1995, Nganampa Health Council (NHC) implemented a comprehensive control program for sexually transmitted infections (STIs) in remote communities on the Anangu Pitjantjatjara Yankunytjatjara (APY) Lands in Central Australia. The program incorporated aggressive screening for gonorrhoea and chlamydial infection, and a report of its first 4 years of operation documented a reduction in prevalence of each of these infections, which was statistically significant in the case of gonorrhoea.1
Ethical approval for the study was granted by the NHC Aboriginal board of management.
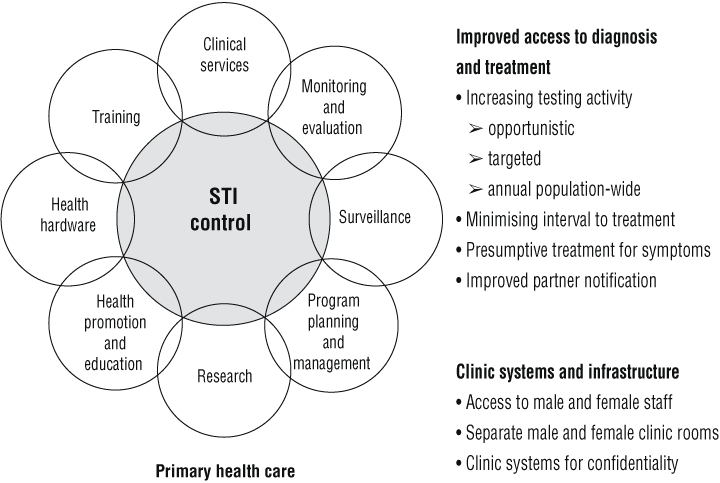
The structure of the comprehensive program is illustrated by the model “Eight ways to beat HIV” (Box 1). The major strategies were developed with guidance from Aboriginal steering committees, who were informed about current international STI control strategies and how they might be adapted locally.
A major focus was on improving diagnosis and treatment of STIs by increasing opportunistic testing throughout the year and conducting annual population-wide screening. Annual syphilis screening had been undertaken since the mid 1980s, resulting in a reduction in syphilis prevalence from 20% in 1985 to 1.7% in 1996.2 The comprehensive program emphasised training staff to recognise symptomatic presentations where treatment, testing and contact tracing were indicated on the day. In addition, education was provided about STIs and risk reduction, and a range of educational materials were developed in the local language.
Diagnosis of gonorrhoea and chlamydial infection was by polymerase chain reaction (PCR) testing of first-void urine for males during all annual screens. For females, self-obtained low vaginal swabs were preferred as samples from 2001, because of the higher sensitivity for gonorrhoea diagnosis.3,4 The supplementary gonorrhoea PCR test varied over the period, but the screening test remained the same (Amplicor PCR, Roche, Branchburg, NJ, USA), and its results are presented here.
Before the 2006 annual population-wide screen, selected NHC clinic staff were trained to inoculate low vaginal swabs in Amies medium and urine sediment onto GC agar plates (Oxoid Australia, Adelaide, SA). Plates were incubated in a high CO2 atmosphere in candle jars and forwarded to Medvet Laboratories, Alice Springs, for further incubation, interpretation and antibiotic sensitivity testing of gonococcal isolates.
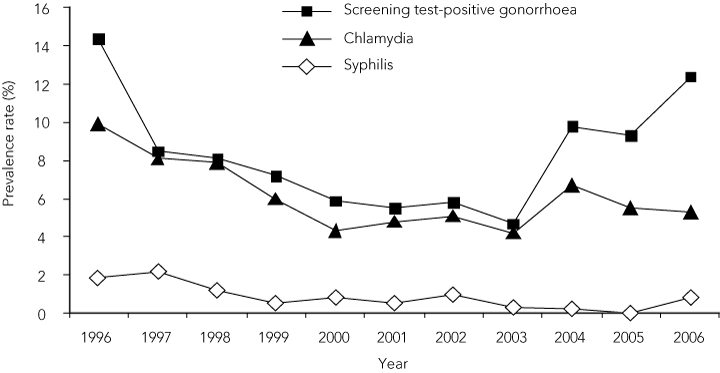
Between 1996 and 2003, the age-adjusted prevalence rates of gonorrhoea and chlamydial infection decreased by 67% and 58%, respectively (Box 2). The decrease in prevalence of gonorrhoea was more rapid at the beginning of the period. After 2003, the prevalence of gonorrhoea increased significantly (Box 3).
For chlamydial infection, there was strong evidence of a reduction in prevalence between 1996 and 2003, with the odds of infection decreasing 12% each year, but no evidence of a change between 2003 and 2006. Syphilis prevalence declined linearly from 1996 to 2006 (Box 3).
The audit of clinical care between 1996 and 2006 showed consistently high participation and treatment rates, and short intervals to treatment in the annual screening, as well as large numbers of interval screens (Box 4).
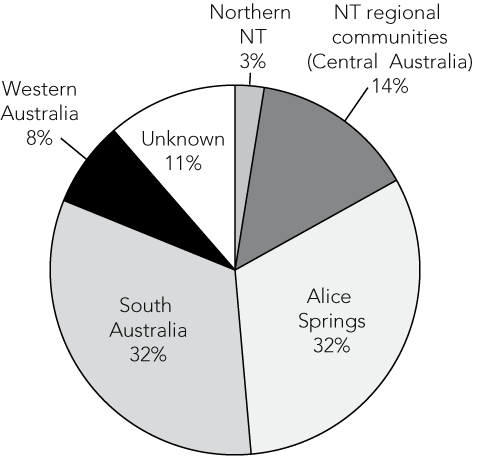
In 2005 and 2006, 28% of the target population was reported to be travelling outside the APY lands in the first 2 weeks of the annual STI screen, mainly to SA and Alice Springs (Box 5).
The study found a significant reduction in prevalence, and sustained control of chlamydial infection between 1996 and 2006. The gonorrhoea prevalence rate also significantly decreased between 1996 and 2003, but this was followed by a sharp rise, despite maintenance of excellent case management. A major reduction in syphilis prevalence was recorded before this study began, largely as a result of annual screening,2 and a further significant linear reduction was achieved over the study period.
In Australia as a whole, gonorrhoea diagnoses in Indigenous people increased by 48% between 2002 and 2006, and chlamydial diagnoses by 61%.5 For meaningful interpretation of our results, it is necessary to consider how much prevalence rates would have risen without the comprehensive program. While there are few published data comparable with our population-wide data, population-wide STI screening was also recently undertaken in selected communities in regions of the Northern Territory adjacent to the APY Lands, assisted by the Tri-State STI/HIV Project. The project reported higher prevalence rates for gonorrhoea than for chlamydial infection among young people between 2001 to 2005,6 with prevalence rates of 14% and 10% reported in 2004 for gonorrhoea and chlamydial infection, respectively, among 15–35-year-olds.7
The gonococcal genome is hypervariable, allowing it to change continually to avoid host immune responses,8 and contributing to its greater outbreak potential compared with chlamydial infection. According to “core transmitter theory” for gonorrhoea,9 there may be frequent interaction within a core group of people, but because a significant proportion are already infected, a rise in new cases is limited by preemption (ie, the disease is already present).9 By 2003, after 8 years of population-wide screening and comprehensive STI control, gonorrhoea prevalence rates had fallen by 67% in the APY Lands. This left an increased population susceptible to acquiring infection because of their negative status. One explanation for the rapid increase in gonorrhoea in 2004 is an increase in travel of core transmitters or bridge populations susceptible to infection to and from regions that lacked comprehensive STI control programs. This implies that health promotion and education had failed to create behaviour change among this group.
There is some evidence of considerable intercommunity and community-to-regional centre mobility among Indigenous people in the NT10 and SA. The yield of positive gonorrhoea tests in the APY Lands in the second half of 2003 was 4.3% (95% CI, 3.0%–5.5%). In the first quarter of 2004, just before the first annual screening that found a rise in gonorrhoea prevalence, the test yield rose to 9.8% (95% CI, 7.0%–12.6%). During this period, over 1000 non-residents were estimated to be camped on the APY Lands for over a month to participate in ceremonial activity, in what was regarded as the largest congregation of this type for a decade.
Another contributing factor to the sudden rise in gonorrhoea might have been the emergence of a gonococcal clone with greater virulence and infectivity. Molecular studies have suggested that a small number of successful gonococcal subtypes with superior virulence factors dominate and can then persist for a considerable period within particular patient groups.11,12 A rise in prevalence through this mechanism would depend on the presence of sexual partners from outside the usual network (facilitated by mobility) and lack of behaviour change among core transmitters allowing the introduced clone to become endemic.
To investigate further the rise in gonorrhoea prevalence, we intend to undertake molecular testing of gonococcal strains obtained during the 2006 culture survey. These techniques have been used to evaluate transmission chains in other settings.11,13,14
There are significant opportunities for STI control among Indigenous people in Australia, given the development of PCR testing for chlamydia and gonorrhoea and culturally acceptable sampling techniques.3,15 These allow intensive screening and treatment, even in remote settings. Both chlamydial infection and gonorrhoea remain curable by single-dose antibiotics in central Australia. In contrast, PCR testing is largely unavailable in other areas of the world with high gonorrhoea prevalence because of the significant costs, necessitating greater reliance on a syndromic approach. There is also high penicillin resistance among gonococci in these areas. However, the hypervariability of the gonococcus and its outbreak potential mean sustained long-term control will probably require a broad reduction in prevalence across interrelated Indigenous populations. While fundamental behaviour change is clearly necessary, in the shorter term, successful control of STIs in Indigenous communities may well require the implementation of comprehensive STI control programs with a focus on increasing testing activity across more regions of remote Australia.
2 Age-adjusted prevalence rates of chlamydial infection, gonorrhoea and syphilis among 14–40-year-olds on the APY Lands, 1996–2006
 | |||||||||||||||
3 Multivariable logistic regression analysis of prevalence of sexually transmitted infections among 14–40-year-olds on the APY Lands, 1996–2006
- Rae-Lin Huang1
- Paul J Torzillo1,2
- Vivien A Hammond1
- Stephanie T Coulter3
- Adrienne C Kirby4
- 1 Nganampa Health Council, Alice Springs, NT.
- 2 Royal Prince Alfred Hospital, Sydney, NSW.
- 3 Infectious Diseases Laboratory, Institute of Medical and Veterinary Science, Adelaide, SA.
- 4 NHMRC Clinical Trials Centre, University of Sydney, Sydney, NSW.
This article is published with permission of the NHC Aboriginal board of management. We thank NHC clinic staff for their contribution, in particular Dr Kerrie Gell for her significant ongoing contribution to STI data management and clinical care. Thanks to Professor Anthony Keech (NHMRC Clinical Trials Centre, Sydney, NSW) for his assistance with statistical analysis. The NHC STI Control and HIV Prevention Program is funded by the Office for Aboriginal and Torres Strait Islander Health, Commonwealth Department of Health and Ageing.
None identified.
- 1. Miller PJ, Torzillo PJ, Hateley W. Impact of improved diagnosis and treatment on prevalence of gonorrhoea and chlamydial infection in remote Aboriginal communities on Anangu Pitjantjatjara Lands. Med J Aust 1999; 170: 429-432.
- 2. Miller PJ, Torzillo P, Tizard J, Winslow B. Interventions to reduce the interval to treatment in syphilis: central case management and encrypted email. Venereology 1998; 11: 26-29.
- 3. Garrow SC, Smith DW, Harnett GB. The diagnosis of chlamydia, gonorrhoea, and trichomonas infections by self obtained low vaginal swabs, in remote northern Australian clinical practice. Sex Transm Infect 2002; 78: 278-281.
- 4. Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis 2002; 29: 647-654.
- 5. National Centre in HIV Epidemiology and Clinical Research. Bloodborne viral and sexually transmitted infections in Aboriginal and Torres Strait Islander people: surveillance report 2007. Sydney: NCHECR, University of New South Wales, 2007.
- 6. Latif AS, Smith KS. Sexually transmitted infections in central Australia — time for concerted action. Public Health Bull S A 2006; 4: 32-33.
- 7. Latif AS, Smith KS. STI screening conducted in NT Department of Health and Community Services and Community Controlled Health Services in Central Australia in 2004. NT Disease Control Bull 2004; 11 (4): 18-20.
- 8. Sparling PF, Tsai J, Cornelissen CN. Gonococci are survivors. Scand J Infect Dis Suppl 1990; 69: 125-136.
- 9. Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhoea. Sex Transm Dis 1978; 5: 51-56.
- 10. Warchivker I, Tapangati T, Wakerman J. The turmoil of Aboriginal enumeration: mobility and service population analysis in a central Australian community. Aust N Z J Public Health 2000; 24: 444-449.
- 11. Choudery B, Risley CL, Ghani AC, et al. Identification of individuals with gonorrhoea within sexual networks: a population-based study. Lancet 2006; 368: 139-146.
- 12. Sarafian SK, Knapp JS. Molecular epidemiology of gonorrhoea. Clin Microbiol Rev 1989; 2 Suppl: S49-S55.
- 13. Kolader M-E, Dukers NH, van der Bij AK, et al. Molecular epidemiology of Neisseria gonorrhoeae in Amsterdam, The Netherlands, shows distinct heterosexual and homosexual networks. J Clin Microbiol 2006; 44: 2689-2697.
- 14. Ward H, Ison CA, Sophie ED, et al. A prospective social and molecular investigation of gonococcal transmission. Lancet 2000; 356: 1812-1817.
- 15. Skov SJ, Miller P, Hateley W, et al. Urinary diagnosis of gonorrhoea and chlamydia in men in remote aboriginal communities. Med J Aust 1997; 166: 468-471. <MJA full text>





Abstract
Objective: To assess the impact of a long-term comprehensive control program for sexually transmitted infections (STIs) in remote Aboriginal communities in Central Australia, and to investigate a recent rise in gonorrhoea prevalence.
Design: STI prevalence was determined from annual, cross-sectional, population-wide, age-based screening, 1996–2006. During 2006, gonococcal isolates were obtained by on-site culture and tested for antimicrobial susceptibility.
Setting: Six remote clinics on the Anangu Pitjantjatjara Yankunytjatjara (APY) Lands, South Australia, which are served by Nganampa Health Council, an Aboriginal community-controlled health service.
Participants: All resident Aboriginal people aged 14–40 years at the commencement date of each annual population-wide screen.
Main outcome measures: Multivariable logistic regression models were used to compare prevalence of chlamydial infection, gonorrhoea and syphilis measured during each annual population-wide screen; antimicrobial susceptibility of gonococcal isolates obtained in 2006.
Results: Between 1996 and 2003, there was a significant reduction in prevalence of gonorrhoea and chlamydial infection, by 67% and 58%, respectively. Subsequently, chlamydia prevalence rate plateaued, but there was a rapid rise in prevalence of gonorrhoea. Syphilis prevalence decreased linearly over the study period (odds ratio, 0.81; P < 0.001). During the first 6 months of 2006, 89 gonococcal isolates were obtained, 39 through on-site culture during the 6-week screening period, and all were sensitive to penicillin (in the less-sensitive category).
Conclusions: The decrease in STI prevalence asssociated with the program was maintained until 2006 for chlamydial infection and syphilis, but not for gonorrhoea, which rose in prevalence after 2003. There was no change in antimicrobial resistance to explain this rise, and gonorrhoea transmission dynamics and travel of core transmitters to regions without STI control programs might be responsible.