Pyomyositis is a primary acute bacterial infection of the large skeletal muscles, associated with abscess formation. Generally more common in tropical regions, pyomyositis also occurs in temperate regions, although its incidence there has not been determined. Mortality of up to 10% in temperate regions has been reported.1 A review of 100 North American cases over 20 years indicated some of the epidemiological differences between temperate and tropical pyomyositis.2 Predisposing conditions included: a recent history of trauma (in up to 63% of cases),2 recent skin infection,2 diabetes mellitus,3 HIV infection,4 injecting drug use5 and other types of immunosuppression.6 Bacteraemia has been reported in up to 31% of cases.2 Importantly, magnetic resonance imaging (MRI) aids early diagnosis and enables identification of patients suitable for percutaneous drainage.7-9
With the increase in the number of strains of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and in the number of Panton–Valentine leukocidin (PVL) secreting strains of methicillin-sensitive S. aureus (MSSA) in our community, early detection of and appropriate therapy for pyomyositis will be important. In temperate-climate disease, clinical features on presentation include pain, fever, limitation of movement of the affected area and associated leukocytosis. Management has comprised intravenous antibiotics with drainage of any abscess, either under radiological guidance or by open procedure. Outcomes in most large series of people with pyomyositis were complete cure, with minimal residual symptoms.1
Blood cultures were incubated in a BACTEC 9120 series continuous monitoring blood culturing instrument (Becton-Dickinson Microbiology Systems, Sparks, Md, USA) and isolates were identified by the MicroScan WalkAway 96 system (Dade Behring Sacramento, Calif, USA). Antibiotic susceptibility testing was tested by MicroScan automated broth dilution or by the Calibrated Dichotomous Sensitivity (CDS) disc diffusion method.10
Incidence rates for pyomyositis were calculated within the Barwon Statistical Division and quoted per 100 000 person-years. Age and sex proportions were also standardised to the Australian population.11
Patients of all ages were affected, and five of our 11 patients (45%) were aged 16 years or younger (range, 6–65 years). Eight of the 11 patients were male (the male-to-female ratio reported in the literature varies between 1:1.5 and 3:1).12 Five patients had an underlying skin disease, and six had engaged in recent vigorous exercise. None of the 11 patients had a history of intravenous drug use, type 2 diabetes or recent trauma. Two patients required computed tomography-guided or ultrasound-guided aspiration of collections, and all patients were completely cured (Box 1).
The detailed pathogenesis of staphylococcal pyomyositis is not known. In 1904, it was shown that pyomyositis could only occur in S. aureus-inoculated animal muscles that had been initially traumatised by electric shock, pinching or ischaemia,13 suggesting the role of initial trauma in the pathogenesis. Vigorous muscle activity, such as in competitive sport, also appears to have a role in pyomyositis,14 and had been undertaken by six of the 11 patients in our series. It is postulated that initial muscle trauma followed by a breach in the skin or mucosa, leading to bacteraemia and ultimately infection of the traumatised muscle, is the pathogenesis.13 Interestingly, none of the patients in our series had a history of trauma. Five patients had an underlying skin disease (three had active dermatitis, one had paronychia and one had eczema) providing a possible portal of entry for the organism. Tropical pyomyositis studies report coexisting pyoderma in 55%–72% of cases.15
All 11 patients in our study presented with severe local pain and restriction of movement, and a fever of 38°C or above, compared with another study reporting only 59% of patients with a temperature of over 38°C at presentation.2
All 11 patients had bacteraemia, with eight having osteomyelitis, one having septic arthritis and one having mitral valve endocarditis as complications. It is unclear whether the bacteraemia precedes or is a result of the pyomyositis. All patients recovered completely with no residual signs of disease, which we hypothesise may have been the result of early detection and institution of appropriate antibiotic therapy.
We did not perform PVL testing, but this may become important with increasing rates of community-associated MRSA and PVL-positive MSSA being reported, and is a possible subject for further research.16
Diabetes mellitus is an important risk factor for the development of pyomyositis, with an increased rate of skin colonisation by S. aureus in patients with diabetes compared with control patients without diabetes.17 None of the patients in our series had elevated fasting plasma glucose levels, which may have contributed to their favourable outcomes. None of our patients had any risk factors for HIV,4 and therefore formal testing was not performed. Pyomyositis has also been associated with injecting drug use,5 malignancy2 and autoimmune disease6, none of which were reported by patients in our series.
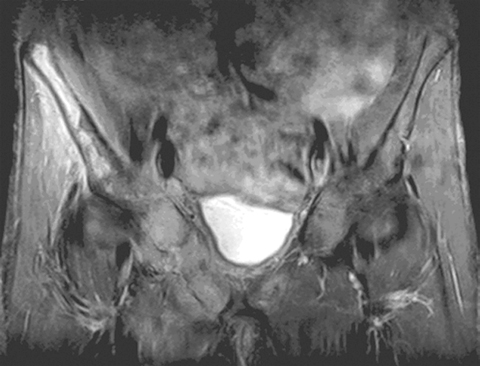
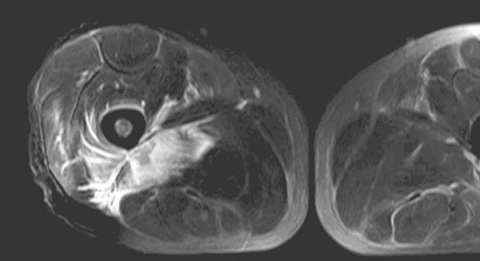
MRI helped in making the diagnosis and delineating the extent of the muscle involvement in all patients, as (see Box 2) the high signal intensity of the pathological process (prolonged T2) can be easily distinguished from the relatively low signal intensity of normal muscle (shortened T2). The superiority of MRI for differentiating pyomyositis from other pathological processes, outlining the extent of involvement and localising fluid collections, has been previously reported.8 The ability of MRI to obtain multiplanar contiguous sections provides excellent anatomical detail of each muscle group and precisely locates the site of disease. MRI scans in 43 cases of pyomyositis found that hyperintense signals on T2-weighted images were detected in all patients.18 A hyperintense rim on unenhanced T1-weighted images and peripheral enhancement after gadolinium injection was useful for identifying the number, size and location of soft tissue abscesses.18
1 Summary of clinical course and outcome of 11 patients with staphylococcal pyomyositis* in Geelong over 110 months
- Andrew A Block1
- Catherine Marshall2
- Alison Ratcliffe3
- Eugene Athan4
- 1 Department of Medicine, Dandenong Hospital (Southern Health), Dandenong, VIC.
- 2 Royal Darwin Hospital, Darwin, NT.
- 3 Royal Hobart Hospital, Hobart, TAS.
- 4 Barwon Health, Geelong Hospital, Geelong, VIC.
We acknowledge the contribution of Stephen Graves in the preparation of this manuscript.
None identified.
- 1. Gibson RK, Rosenthal SJ, Lukert BP. Pyomyositis: increasing recognition in temperate climates. Am J Med 1984; 77: 768-772.
- 2. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis 1992; 15: 668-677.
- 3. Walling DM, Kaelin WG. Pyomyositis in patients with diabetes mellitus. Rev Infect Dis 1991; 13: 797-802.
- 4. Al-Tawfiq JA, Sarosi GA, Cushing HE. Pyomyositis in the acquired immunodeficiency syndrome. South Med J 2000; 93: 330-334.
- 5. Hseuh P, Hsiue TR, Hsieh WC. Pyomyositis in intravenous drug abusers: report of a unique case and review of the literature. Clin Infect Dis 1996; 22: 858-860.
- 6. Minor RL, Baum S, Schulze-Delrieu KS. Pyomyositis in a patient with progressive systemic sclerosis. Arch Intern Med 1988; 148: 1453-1455.
- 7. Meena AK, Rajashekar S, Reddy JJ, et al. Pyomyositis — clinical and MRI characteristics report of three cases. Neurol India 1999; 47: 324-326.
- 8. Yuh WT, Schreiber AE, Montgomery WJ, Ehara S. Magnetic resonance imaging of pyomyositis. Skeletal Radiol 1988; 17: 190-193.
- 9. Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology 1995; 197: 279-286.
- 10. Bell SM. The CDS method of antibiotic sensitivity (calibrated dichotomous sensitivity testing). Pathology 1975; 7 (4 Suppl): S1-S48.
- 11. Australian Bureau of Statistics. 2001 Census QuickStats: Barwon (Statistical Division). Person characteristics; Family characteristics; Dwelling characteristics. http://www.censusdata.abs.gov.au/ (accessed Aug 2008).
- 12. Chiedozi LC. Pyomyositis: review of 205 cases in 112 patients. Am J Surg 1979; 137: 255-259.
- 13. Miyake H. [Beitrage zur Kenntnis der sogenannten Myositis infectiosa]. Mitt Grenzgeb Med Chir 1904; 13: 155-198.
- 14. Jayoussi R, Bialik et al. Pyomyositis caused by vigorous exercise in a boy. Acta Paediatr 1995; 84: 226-227.
- 15. Aderele WI, Osinusi K. Pyomyositis in childhood. J Trop Med Hyg 1980; 83: 99-104.
- 16. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2006; 43: 953-960.
- 17. Tuazon CU, Perez A, Kishaba T, Sheagren JN. Staphylococcus aureus among insulin injecting diabetic patients. An increased carrier rate. JAMA 1975; 231: 1272.
- 18. Soler R, Rodriguez E, Aguilera C, Fernandez R. Magnetic resonance imaging of pyomyositis in 43 cases. Eur J Radiol 2000; 35: 59-64.





Abstract
Objectives: To describe all cases of staphylococcal pyomyositis in the Geelong region of Victoria over 110 months, to estimate the incidence of this disease, and to describe the clinical outcomes and identify any predisposing factors.
Design, participants and setting: A prospective case series identified by clinical features (local pain and fever) and magnetic resonance imaging (MRI) findings (hyperintense signal on T2-weighted scan), among patients presenting to Geelong Hospital, Victoria between 1 April 1998 and 1 June 2007.
Main outcome measures: Estimation of incidence, clinical course and identification of predisposing factors.
Results: We estimate an annual incidence of 0.5 cases per 100 000 person-years, and propose a recent history of vigorous exercise (six of 11 patients) and underlying skin condition (five of 11 patients) as possible predisposing factors. MRI showed eight patients had osteomyelitis and one had septic arthritis. All patients had bacteraemia and one had mitral valve endocarditis. The duration of intravenous antibiotic therapy varied between 4 and 12 weeks, and all patients were completely cured.
Conclusion: Pyomyositis should be considered in patients presenting with local pain, fever, muscle tenderness, and a recent history of vigorous exercise or underlying skin condition. MRI may guide non-surgical management.