Clinical record
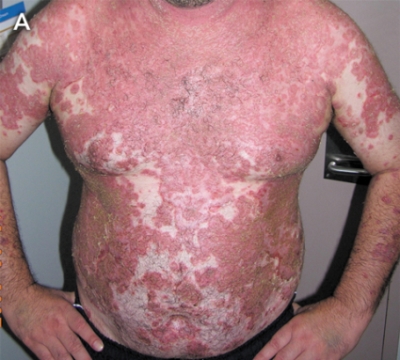
A 44-year-old Samoan man presented in 2004 with a blistering skin rash affecting his face, trunk and upper limbs (Figure, A). There was no mucosal involvement and a positive Nikolsky sign (the production of a blistering lesion on applying pressure to non-affected skin) was demonstrated. Skin biopsy revealed acantholysis and cleft formation within the superficial layers of the epidermis, confirming a diagnosis of pemphigus foliaceus. Direct immunofluorescence testing of a perilesional skin biopsy specimen revealed deposition of IgG and C3 in the intercellular cement substance of all layers of the epidermis. Indirect immunofluorescence testing, using a monkey oesophagus substrate, demonstrated a high titre of autoantibodies in the patient’s serum.
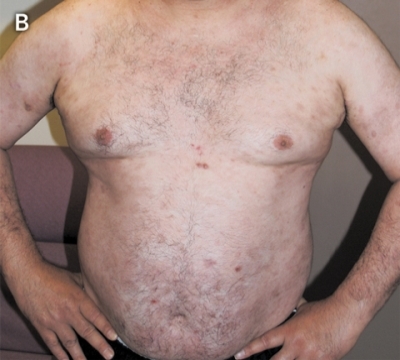
The hospital drug committee approved off-label use of two courses of rituximab (375 mg/m2). This resulted in rapid resolution of the lesions and a marked decrease in antipemphigus antibodies. Depletion of B cells was evident on immunophenotyping of peripheral blood mononuclear cells, and the corticosteroid dose was subsequently weaned to 0.1 mg/kg/day. Nine months after treatment with rituximab, the patient’s clinical condition remained stable (Figure, B).
Autoimmune blistering diseases are rare but potentially life-threatening.1 Pemphigus foliaceus is characterised by superficial erosion of the skin without mucosal involvement. The disease is mediated by autoantibodies directed against desmoglein-3,2 a structural protein involved in epidermal cell adhesion. The levels of these pathogenic autoantibodies can be measured by indirect immunofluorescence and, as illustrated in our patient, are useful for monitoring disease activity.2 The combination of prednisone and azathioprine is often used as first-line therapy,3 but other immunosuppressive agents, as well as intravenous immunoglobulins and plasmapheresis, have also been used to control disease activity.4 However, these therapies do not induce remission in all cases and they can be associated with significant adverse events.
Lessons from practice
Blistering skin diseases are potentially life-threatening and can have an autoimmune basis.
Direct immunofluorescence of a perilesional skin biopsy is essential for diagnosis.
Titres of autoantibodies in the patient’s serum, measured by indirect immunofluorescence, are useful for monitoring disease activity.
Rituximab can be used successfully for the treatment of severe, refractory pemphigus foliaceus.
Rituximab is a chimeric (human/murine) monoclonal antibody directed against CD20, a cell surface molecule specific to B cells. Although it was initially approved for use in B-cell non-Hodgkin’s lymphoma, a growing number of reports have described the efficacy of rituximab for B-cell depletion in the treatment of autoimmune diseases.5 The mode of action of rituximab in autoimmune diseases may include removal of the precursors of autoantibody-producing plasma cells and impairment of autoantigen presentation to T cells.6 A recent case series showed that rituximab induced remission in 18 of 21 patients with pemphigus, whose condition had not responded or who had contraindications to corticosteroid therapy. The treatment was generally well tolerated; however, two cases were complicated by severe infection, one resulting in death.7
Ongoing surveillance of patients treated with rituximab for pemphigus and other autoimmune diseases is required to monitor long-term complications. A recent review of the increasing use of rituximab for off-label indications in a large teaching hospital highlighted the need for a database to help determine optimal dosing regimens and to record clinical outcomes.8
- Suran L Fernando1
- Kate S O’Connor2
- 1 Department of Clinical Immunology and Allergy, Royal North Shore Hospital, Sydney, NSW.
- 2 Department of Immunology, Royal Victoria Infirmary, Newcastle-upon-Tyne, UK.
- 1. Stanley JR, Amagai M. Pemphigus, bullous impetigo and Staphylococcal scalded skin syndrome. N Engl J Med 2006; 355: 1800-1810.
- 2. Gniadecki R. Desmoglein autoimmunity in the pathogensis of pemphigus. Autoimmunity 2006; 39: 541-547.
- 3. Wood AJ, Wood MD. Management of acquired bullous skin diseases. N Engl J Med 1995; 333: 1475-1484.
- 4. Dick SE, Werth VP. Pemphigus: a treatment update. Autoimmunity 2006; 39: 591-599.
- 5. Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov 2006; 5: 564-576.
- 6. Edwards JC, Cambridge G. Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology 2005; 44: 151-156.
- 7. Joly P, Mouquet H, Roujeau JC, et al. Single cycle of Rituximab for the treatment of severe pemphigus. N Engl J Med 2007; 357: 545-552.
- 8. Sharma R, Koller L, Barcaly P, Liddle C. Evaluation of the off-label usage of Rituximab in a large teaching hospital in New South Wales. Intern Med J 2007; 37: 569-571.




