In Western populations, venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common cause of morbidity and one of the most common preventable causes of inhospital deaths.1
As no community-based study has been performed in Australia, reliable data on the local incidence of VTE are lacking. International incidence figures may not be generalisable to the Australian population, as studies from other countries have shown regional variations. For example, VTE incidence in the Brest district of France was found to be almost twice that in north-eastern England,2,3 and, in Sweden, the incidence of DVT in two districts in the 1970s was half that in the city of Malmö in 1987.4,5
This was a prospective, community-based study with multiple overlapping sources of case ascertainment, similar to the Perth Community Stroke Study (PCSS) conducted in 1986.6 We conducted this study from 1 October 2003 to 31 October 2004.
The study population comprised all residents of an area of about 94 km2 in north-eastern metropolitan Perth (defined by 12 postcodes: 6000, 6003, 6004, 6006, 6050–6054, 6059, 6060 and 6062). This represented an expansion of the area covered by the PCSS, which included eight complete postcode districts and part of a ninth.6 Royal Perth Hospital, a public tertiary referral centre, is located toward the southern apex of the study area, and another public tertiary centre, Sir Charles Gairdner Hospital, is situated just outside of the area to the west.
Retrospectively, hospital patients were identified by searching the hospital morbidity and mortality databases of the Western Australian Department of Health. All public and private hospitals in the Perth area are required to submit hospital discharge data to the Department of Health, where they are regularly collated. We retrieved data on VTE hospitalisations using ICD-10 (International classification of diseases, 10th revision) codes (Box 1).7
When previous medical records and radiology reports were unavailable, and there was no satisfactory clinical or diagnostic tool to confirm a previous diagnosis of VTE, we applied two sets of questions validated for use in epidemiological studies8 to ascertain: (1) if patients thought they had ever had a VTE, if they had ever been hospitalised for a PE, or if a physician had ever diagnosed them with a VTE; and (2) if they had ever received anticoagulation therapy. We considered patients answering in the affirmative to both sets of questions to have had previous VTE, and the index event was then counted as a recurrence. This approach has a sensitivity of 37.1%, specificity of 99.4%, and positive and negative predictive values of 48.9% and 98.5%, respectively.8
Age-adjusted annual incidence of VTE was the number of patients with symptomatic, objectively verified VTE per 1000 residents in the study area per year adjusted (according to age) to the World Health Organization World Standard Population,9 calculated by direct standardisation.10
We calculated 95% confidence intervals around estimates using exact limits.11
During the study period, there were 140 VTE events among 137 patients (Box 2). Sixty-six patients with VTE (48.2%) were identified during hospital presentation or at the Royal Perth Hospital Thrombosis Clinic, and 22 (16.1%) were identified through review of radiology lists. Thirty-nine patients (28.5%) were ascertained from the hospital morbidity and mortality databases; four (2.9%) from domiciliary nursing programs; two (1.5%) by referral to the study by other nurses; two (1.5%) by referral from a private radiological service; one (0.7%) from the Coroner’s Court; and one patient (0.7%) identified himself to the study after seeing a local advertisement.
The crude annual incidence per 1000 residents was 0.83 (95% CI, 0.69–0.97) for VTE (Box 2), 0.52 (95% CI, 0.41–0.63) for DVT, and 0.31 (95% CI, 0.22–0.40) for PE. The annual incidences per 1000 residents adjusted to the WHO World Standard Population were 0.57 (95% CI, 0.47–0.67), 0.35 (95% CI, 0.26–0.44) and 0.21 (95% CI, 0.14–0.28) for VTE, DVT and PE, respectively.
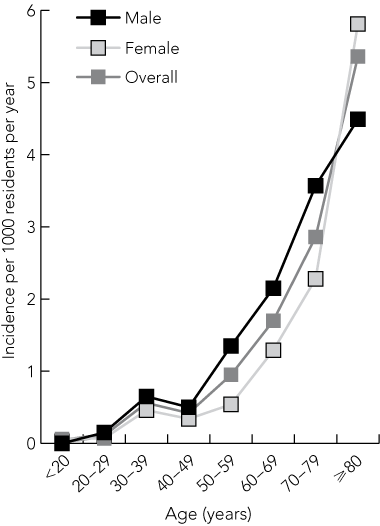
There was only one case of VTE among patients aged < 20 years; in this age group, the annual incidence was 0.03 (95% CI, 0–0.09) per 1000 residents. Among patients aged ≥ 80 years, the annual incidence was 5.36 (95% CI, 3.59–7.13) per 1000 residents (Box 3).
This study has some limitations that may have resulted in underestimation of the number of cases of VTE. Quantifying the completeness of ascertainment is difficult, but it is unlikely that we identified every case of objectively verified, symptomatic VTE. PE can be difficult to diagnose antemortem, and fatal cases may have been missed due to very low autopsy rates. At Royal Perth Hospital and Sir Charles Gairdner Hospital, the autopsy rates during the study period were only 4.3% (41/955) and 3.4% (29/852), respectively, for all deaths that were not the subject of a coronial inquiry. Therefore, it is likely that our results underestimate the true burden of disease. The trend away from postmortem examinations appears to be a worldwide phenomenon.2,3,12,13
Some symptomatic patients would have been excluded because their VTE was not objectively verified. Furthermore, the limited sensitivity of ultrasonography in detecting distal lower limb DVT14 and the exclusion of all cases with “intermediate” or “low” probability for PE on ventilation/perfusion scanning mean that some true cases were not counted.15
Patients with DVT (especially those with distal limb thrombosis) managed in the community by GPs and not referred to this study could have been missed. It is unlikely that a significant number of patients with PE were managed in the community and missed by our study because home-based treatment was not recommended at the time,16 although data have since accumulated to support safety and validation of outpatient management for selected cases of PE.17,18
Between the PCSS in 19866 and the census in 2001, north-eastern metropolitan Perth recorded an annual average population growth of 0.85%. Extrapolating the 2001 census data to 2003 gives an annual VTE incidence of 0.82 per 1000 residents.
Comparing VTE incidence across studies is difficult due to differences in study methods (eg, non-verification of all symptomatic cases by objective means; inclusion of asymptomatic cases; inclusion of only cases of DVT and not PE) and changes in diagnostic methods over time. Current diagnostic tests are more convenient and less invasive than methods used in the past (eg, venography and pulmonary angiography) but have limited sensitivity in some instances. Further, the age structures of populations differ, so crude incidence data should be adjusted to a nominal standard (eg, the WHO World Standard Population9) for meaningful comparison across studies.
Consistent with other community-based incidence studies,2,4,5 we found that VTE incidence increases with age. However, we were unable to confirm a previous report that the incidence of PE increases (as a proportion of VTE cases) with age,2 as the mean age of patients with DVT in our cohort was similar to those with PE.
The crude annual incidence of VTE we found for a community in Perth is similar to that reported in north-eastern England (0.96 [95% CI, 0.91–1.01])3 but lower than that reported in studies from Europe.2,4,5,19 We believe the differences in incidence between Perth and parts of Europe may reflect differences in ethnicity (eg, the prevalence of the most common inherited thrombophilia, factor V Leiden, is only 2%–4% in Perth20,21 compared with 15% in southern Sweden22) and cultural and environmental factors (eg, obesity, and clinical practices in regard to thromboprophylaxis and female hormonal manipulation).
An alternative explanation is that the lower incidence in our study is due to under-ascertainment of cases. We believe this is less likely, as case ascertainment was optimised by using multiple overlapping sources, prospectively and retrospectively. Another possible explanation for the lower VTE incidence in Perth is the low autopsy rate (particularly compared with Malmö, Sweden, where autopsies were performed in 79% of all deaths in 19875), which predisposes to suboptimal detection of fatal PE. However, recent community-based studies in Europe2 also had low autopsy rates and limited access to coronial data, yet found a higher VTE incidence than in Perth.
1 ICD-10 codes used to retrospectively identify cases of venous thromboembolism
I80.1: Phlebitis and thrombophlebitis of femoral vein
I80.2: Phlebitis and thrombophlebitis of other deep vessels of lower extremities
I80.3: Phlebitis and thrombophlebitis of lower extremities, unspecified
I80.8: Phlebitis and thrombophlebitis of other sites
I80.9: Phlebitis and thrombophlebitis of unspecified site
I82: Other venous embolism and thrombosis
O07.2: Failed medical abortion, complicated by embolism
O07.7: Other and unspecified failed attempted abortion, complicated by embolism
O08.2: Embolism following abortion and ectopic and molar pregnancy
O22.3: Deep phlebothrombosis in pregnancy
O22.8: Other venous complications in pregnancy
O22.9: Venous complication in pregnancy, unspecified
O87.1: Deep phlebothrombosis in the puerperium
O87.9: Venous complication in the puerperium, unspecified
O88.2: Obstetric blood-clot embolism
G08: Intracranial and intraspinal phlebitis and thrombophlebitis
K55.0: Acute vascular disorders of intestine
K55.9: Vascular disorders of intestine, unspecified
K75.1: Phlebitis of portal vein
N28.0: Ischaemia and infarction of kidney
ICD-10 = International classification of diseases, 10th revision.7
Received 4 October 2007, accepted 5 February 2008
- Wai Khoon Ho1
- Graeme J Hankey2
- John W Eikelboom3
- 1 Department of Haematology, Austin and Repatriation Medical Centre, Melbourne, VIC.
- 2 Stroke Unit, Royal Perth Hospital, Perth, WA.
- 3 Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Wai Khoon Ho was supported by a scholarship from the Centre of Clinical Research Excellence, University of WA, when conducting this study. We thank the following for their help during this study: Mrs Anne Claxton; Dr John Teasdale and the WA Vascular Centre; Dr David Oldham and the Osborne GP Network for data access from the Home-Ward Programme; Data Linkage Unit, WA Department of Health; Coroner’s Court of WA, WA Department of Justice; Silver Chain; SKG Radiology; Fremantle Hospital; Hollywood Private Hospital; King Edward Memorial Hospital for Women; Mercy Hospital Mount Lawley; Mount Hospital; Royal Perth Hospital; Sir Charles Gairdner Hospital; Swan Health Service; Australian Medical Association (WA); and the GPs, nurses and patients who participated in the study.
None identified.
- 1. Fedullo PF. Pulmonary thromboembolism. In: Murray JF, Nadel JA, Mason RJ, Boushey HA, editors. Textbook of respiratory medicine. 3rd ed. Philadelphia: WB Saunders, 2000: 1503-1531.
- 2. Oger E; EPI-GETBP Study Group. Incidence of venous thromboembolism: a community-based study in Western France. Thromb Haemost 2000; 83: 657-660.
- 3. Kesteven P, Robinson B. Incidence of symptomatic thrombosis in a stable population of 650,000: travel and other risk factors. Aviat Space Environ Med 2002; 73: 593-596.
- 4. Kierkegaard A. Incidence of acute deep vein thrombosis in two districts. A phlebographic study. Acta Chir Scand 1980; 146: 267-269.
- 5. Nordström M, Lindblad B, Bergqvist D, Kjellström T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med 1992; 232: 155-160.
- 6. Ward G, Jamrozik K, Stewart-Wynne E. Incidence and outcome of cerebrovascular disease in Perth, Western Australia. Stroke 1988; 19: 1501-1506.
- 7. World Health Organization. International statistical classification of diseases and related health problems, 10th revision. http://www.who.int/classifications/apps/icd/icd10online (accessed Dec 2006).
- 8. Frezzato M, Tosetto A, Rodeghiero F. Validated questionnaire for the identification of previous personal or familial venous thromboembolism. Am J Epidemiol 1996; 143: 1257-1265.
- 9. Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new WHO standard. GPE Discussion Paper Series No. 31. http://www.who.int/healthinfo/paper31.pdf (accessed Nov 2006).
- 10. Daly LE, Bourke GJ. Multivariate analysis and the control of confounding. In: Interpretation and uses of medical statistics. 5th ed. Oxford: Blackwell Science, 2000: 339-380.
- 11. Daly LE, Bourke GJ. Confidence intervals: general principles; proportions, means, medians, counts and rates. In: Interpretation and uses of medical statistics. 5th ed. Oxford: Blackwell Science, 2000: 86-115.
- 12. Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158: 585-593.
- 13. Bergqvist D. Incidence of pulmonary embolism: is it declining? Semin Vasc Surg 2000; 13: 167-170.
- 14. Fraser JD, Anderson DR. Deep venous thrombosis: recent advances and optimal investigation with US. Radiology 1999; 211: 9-24.
- 15. Kearon C. Diagnosis of pulmonary embolism. CMAJ 2003; 168: 183-194.
- 16. Yusen RD, Gage BF. Outpatient treatment of acute venous thromboembolic disease. Clin Chest Med 2003; 24: 49-61.
- 17. Dager WE, King JH, Branch JM, et al. Tinzaparin in outpatients with pulmonary embolism or deep vein thrombosis. Ann Pharmacother 2005; 39: 1182-1187.
- 18. Siragusa S, Arcara C, Malato A, et al. Home therapy for deep vein thrombosis and pulmonary embolism in cancer patients. Ann Oncol 2005; 16 Suppl 4: iv136-iv139.
- 19. Nylander G, Olivecrona H. The phlebographic pattern of acute leg thrombosis within a defined urban population. Acta Chir Scand 1976; 142: 505-511.
- 20. Hankey GJ, Eikelboom JW, van Bockxmeer FM, et al. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32: 1793-1799.
- 21. van Bockxmeer FM, Baker RI, Taylor RR. Premature ischaemic heart disease and the gene for coagulation factor V. Nat Med 1995; 1: 185.
- 22. Zöller B, Norlund L, Leksell H, et al. High prevalence of the FVR506Q mutation causing APC resistance in a region of southern Sweden with a high incidence of venous thrombosis. Thromb Res 1996; 83: 475-477.





Abstract
Objective: To determine the incidence of venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), in a well defined urban community broadly representative of the Australian population in terms of age, sex and ethnic distribution.
Design, setting and participants: A prospective, community-based study conducted over a 13-month period from 1 October 2003 to 31 October 2004. People in a population of 151 923 permanent residents of north-eastern metropolitan Perth, Western Australia, who developed VTE during the study period were identified prospectively and retrospectively through multiple overlapping sources.
Main outcome measure: Number of cases of symptomatic, objectively verified DVT and PE.
Results: 137 patients had 140 VTE events (87 DVT and 53 PE). The crude annual incidence per 1000 residents was 0.83 (95% CI, 0.69–0.97) for VTE, 0.52 (95% CI, 0.41–0.63) for DVT, and 0.31 (95% CI, 0.22–0.40) for PE. The annual incidence per 1000 residents after age adjustment to the World Health Organization World Standard Population was 0.57 (95% CI, 0.47–0.67) for VTE, 0.35 (95% CI, 0.26–0.44) for DVT, and 0.21 (95% CI, 0.14–0.28) for PE.
Conclusion: If the crude annual incidence of VTE in this area of metropolitan Perth is externally valid, then VTE affects about 17 000 Australians annually. Future studies of trends in VTE incidence will be needed to measure the effectiveness of VTE prevention strategies.