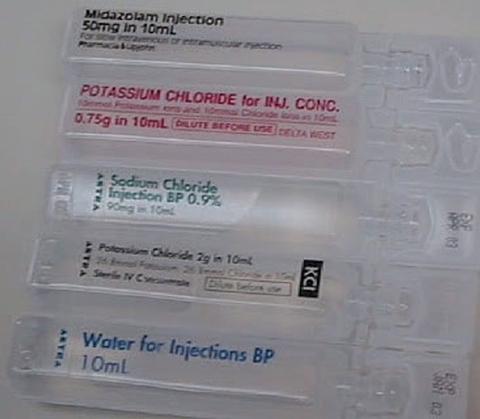
Fatalities due to errors associated with inappropriate bolus injection of potassium chloride (KCl) concentrate from ampoules have been reported in many countries, including Australia.1-5 A search of the National Coroners Information System database in October 2006 identified six deaths from KCl administration in Victoria since 1992, and it is possible that this is an underestimation due to coding errors, missing data or under-reporting. The main reason fatal errors occur is that ampoules of concentrated KCl have a very similar appearance to ampoules of normal saline or water (see Box 1). If injected in place of normal saline to flush an intravenous (IV) line or used instead of water to reconstitute a vial of antibiotic, the bolus effect of the KCl can lead to cardiac arrest.
As a result of such fatalities, several countries have issued medication alerts and made recommendations to improve the safety of IV KCl use.6-8 A 2003 medication alert issued by the Australian Council for Safety and Quality in Health Care stated that the availability of KCl ampoules as medication stock in patient care areas was the root cause of errors.6 The Council’s recommendation was to remove KCl ampoules and replace them with premixed KCl infusions (a manufactured infusion bag containing KCl), which can be administered without any manipulation in a patient care area.
The degree to which this recommendation has been incorporated into practice in Australian hospitals is variable. In some hospitals, removal of KCl ampoules has occurred in general wards9 but not in specialty areas, such as intensive care and haematology/oncology units, as there is no sufficiently concentrated, small-volume premixed KCl infusion available to meet the clinical needs of these patient groups. A survey of 25 Victorian hospitals in 2006 found that 23 had removed ampoules from the general wards, but only five rural hospitals had completely removed ampoules from the hospital.10
Data recorded for all IV KCl prescriptions each day in each audit area were:
number of IV KCl prescriptions;
number of times IV KCl was administered; and
whether premixes or ampoules of concentrated KCl were used.
Prescriptions were classified into three categories:
prescriptions that exactly matched an existing, available premixed infusion;
prescriptions that varied slightly from an existing premixed infusion, which could still have been used for administration (eg, an order for a premixed infusion plus an additive, for an infusion bag with an additive port); and
prescriptions for which no suitable premixed infusion was available. As any prescription for an infusion more concentrated than the existing premix of 10 mmol in 100 mL could only be administered using an ampoule, this category was subdivided into
infusions more concentrated than 10 mmol KCl in 100 mL; and
A total of 888 prescriptions and 1088 IV KCl dose administrations were documented across the six hospitals. There were 69 different types of orders for IV KCl, varying in either concentration or volume. Data on all prescriptions and administrations are shown in Box 2.
Experiences elsewhere show that this problem is not unique to Australia. A recent study reported that 53 different IV KCl infusions were prepared in near-patient clinical areas in hospitals in the north of England.11 This occurred despite a United Kingdom National Patient Safety Agency recommendation that ready-to-use diluted infusions should be used where possible and, if an infusion of the required dilution is not available, it should be prepared in the hospital pharmacy.8
Traditionally, the choice of product for administration of IV fluids and electrolytes has not been considered to be a medical responsibility. A UK study found that doctors were not aware of procedures for storage or recording of IV KCl or obtaining it out of hours, claiming these to be nursing responsibilities.12
Our findings have significant implications for practice. Currently, KCl ampoules remain available in specialty areas in Australian hospitals and, as long as this continues, the risk that another potentially fatal error will occur also remains. Some hospitals have introduced extra safety measures for storing KCl ampoules in specialty areas;13 however, the most effective method of avoiding risk is removal of the hazard.14 This can only occur if suitable premixed infusions are available to replace ampoules.
A number of recommendations related to IV KCl should be considered for adoption. These include:
An expanded range of commercially prepared premixed KCl infusions, including
10 mmol KCl in 100 mL with an additive port, to replace the current equivalent infusion bag that does not have an additive port; and
20 mmol KCl in 50 mL for patients with a central venous catheter;
Improved labelling and packaging of all IV KCl products so they are not confused with other products;
Standardisation of prescribing and administration practices, including
prescribing premixed infusions whenever possible; and
empowering nursing staff and pharmacists to have orders amended so a premix may be used, if appropriate;
Ongoing implementation of education and audit programs at the hospital level, including monitoring of compliance by accreditation bodies; and
Endorsement of these recommendations by key stakeholder groups, such as the Australian and New Zealand Intensive Care Society.
2 Intravenous potassium chloride (KCl) prescriptions and administration
- Melita A Van de Vreede1
- Sally G Wilson1
- Michael J Dooley1,2
- 1 Pharmacy Department, Bayside Health, Melbourne, VIC.
- 2 Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Melbourne, VIC.
This project was supported by an unrestricted grant from Baxter Healthcare. The development, conduct and analysis of the project were independent of Baxter Healthcare. We thank the participating hospitals, their project reference groups and project officers, and Sarah Knight, Alison Whitlock, Amy McRae, Annabel McNally, the summer research students at The Alfred, Dr Chris O’Callaghan, Kent Garrett, Ms Anne McGrath, Dr Mark Lubliner and Dr Michael Bailey for their assistance and support.
None identified.
- 1. National Patient Safety Agency. Patient safety bulletin, January 2007. http://www.npsa.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=6341 (accessed Jun 2007).
- 2. State Coroner of Victoria. Coroner’s report: findings. Coroner’s Case Number 2184/03.
- 3. Tubman M, Majumdar SR, Lee D, et al. Best practices for safe handling of products containing concentrated potassium. BMJ 2005; 331: 274-277.
- 4. Institute for Safe Medication Practices Canada. More on potassium chloride. ISMP Canada Safety Bulletin 2003; 3 (11): 1-2. http://www. ismp-canada.org/download/ISMPCSB2003-11KCl.pdf (accessed Jan 2008).
- 5. Wetherton AR, Corey TS, Buchino JJ, Burrows AM. Fatal intravenous injection of potassium in hospitalized patients. Am J Forensic Med Pathol 2003; 24: 128-131.
- 6. Australian Council for Safety and Quality in Health Care. Medication alert: intravenous potassium chloride can be fatal if given inappropriately. October 2003. http://www.safetyandquality.org/internet/safety/publishing.nsf/Content/E10A5557BF8C10B2CA2571D80004316A/$File/kcalertfinal1.pdf (accessed Jun 2007).
- 7. Joint Commission. Sentinel event alert: medication error prevention — potassium chloride. Issue 1, February 1998. http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_1.htm (accessed Aug 2007).
- 8. National Patient Safety Agency. Patient safety alert: potassium chloride concentrate solutions. (Ref. PSA 01.) London: NPSA, 2002. http://www.npsa.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=5341 (accessed Sep 2007).
- 9. Lubliner M, Van de Vreede MA. Preventing potassium problems: eliminating concentrated potassium chloride errors. J Pharm Pract Res 2006; 36: 181-184.
- 10. Van de Vreede MA, Wilson SG, Dooley MJ. Intravenous potassium chloride use in hospitals — current practice. J Pharm Pract Res 2008; 38: 19-22.
- 11. Hardy L, Mellor L. Risk assessment of parenteral product preparation across secondary care acute trusts in the north of England. Hosp Pharmacist 2007; 14: 58-64.
- 12. Lankshear AJ, Sheldon TA, Lowson KV, et al. Evaluation of the implementation of the alert issued by the UK National Patient Safety Agency on the storage and handling of potassium chloride concentrate solution. Qual Saf Health Care 2005; 14: 196-201.
- 13. Burdeu G, Crawford R, Van de Vreede M, McCann J. Taking aim at infusion confusion. J Nurs Care Qual 2006; 21: 151-159.
- 14. Reason J. Human error: models and management. BMJ 2000; 320: 768-770.





Abstract
Objective: To identify current prescribing and administration practices in relation to intravenous potassium chloride (IV KCl).
Design and setting: A prospective multicentre assessment of IV KCl prescribing and administration at six public hospitals (three large metropolitan hospitals, a smaller metropolitan specialty hospital, and two rural hospitals) in Victoria between August and December 2006. Data were collected for either a 4-week period or for 200 IV KCl orders, whichever occurred first, in clinical areas where concentrated KCl ampoules were available.
Main outcome measures: Number and type of IV KCl prescriptions and dose administrations; method of preparation and administration of each dose.
Results: A total of 888 prescriptions and 1088 administrations were assessed across the six hospitals. There were 69 different types of orders for IV KCl, varying in either concentration or volume. KCl ampoules were used in 59% of all administrations of IV KCl. In instances where the prescription matched an available premixed IV KCl infusion, the premix was used on 89% of occasions.
Conclusions: There is significant variability in the prescribing and administration of IV KCl in these Victorian hospitals. New formulations of premixed IV KCl infusions may enable the removal of ampoules from patient care areas. The medical profession can play a major role in driving the adoption of consistent practice and supporting and leading this important safety initiative.