Tinea, or ringworm, is an infection caused by dermatophyte fungi belonging to the genera Trichophyton, Microsporum and Epidermophyton.1 Tinea capitis is a dermatophyte infection of scalp and hair and can occur in isolation, or with concurrent tinea corporis (body) or tinea unguium (nails). There are two main patterns of tinea capitis infection: ectothrix and endothrix infections.
In ectothrix infections, fungal arthroconidia cover the outside of infected hairs, which eventually break off a few millimetres above the scalp surface, forming circular areas of partial alopecia. Fine scaling is characteristic, and inflammation varies from minimal with human or anthropophilic species (eg, Microsporum audouinii) to moderate or severe with animal or zoophilic (eg, Microsporum canis) or soil-associated geophilic (eg, Microsporum gypseum) dermatophytes.1,2
In endothrix infections, for example those caused by the anthropophilic species Trichophyton tonsurans, Trichophyton violaceum or Trichophyton soudanense, the fungus is confined to the interior of the hair shaft. Infected hairs become very fragile, breaking off level with the follicular orifice and producing “black dot” tinea capitis in patients with dark hair. Hair loss can be minimal, with little inflammation; patients often present with mild seborrhoeic dermatitis-like or dandruff-like scaling.1,2
From the early 1990s, appreciable numbers of African refugees have migrated to Europe, Australia and New Zealand. There have been subsequent reports of tinea capitis in African immigrants caused by T. violaceum, T. soudanense and M. audouinii from a number of European countries3-6 and from Australasia.7-10 Transmission of these infections to the local population is well documented.4,6,10
Clinical examinations were conducted by registrars under the supervision of a consultant dermatologist (A H C). Clinical diagnoses of tinea capitis, corporis and unguium were noted on the screening form. A microbiological specimen was taken from any suspicious scalp, body or nail lesion. Scalp and body lesions were taken using the blunt side of a scalpel; hair stubs were plucked with forceps; nail clippings were taken with nail clippers. The scalp of every participant was also brushed with a scalp massager brush3 (Box 1).
The brushes were pressed onto plates containing Sabouraud glucose agar plus antibacterial agents (gentamicin, chloramphenicol) plus cycloheximide. Part of each hair, skin or nail specimen was used for potassium hydroxide examination (20% KOH plus chlorazol black E, 100 mg/100 mL), and the rest was placed onto slopes of Sabouraud glucose agar plus antibacterial agents with or without cycloheximide. Specimens were incubated at 28°C for 3 weeks. Isolates were identified on the basis of macromorphology, micromorphology and nutritional requirements.
Children were classified as cases, carriers or uninfected on the basis of their clinical appraisal and the isolation of a dermatophyte. A child was considered a case if there was a clinical diagnosis and a dermatophyte was isolated from hairs, skin scrapings, nail clippings or a scalp brush; or in the absence of a clinical diagnosis if a scalp brush gave growth from more than 50% of the inoculum points.3 A child was considered a carrier if there was no clinical diagnosis but a scalp brush gave growth from less than 50% of the inoculum points. Uninfected children had no clinical lesions and no growth from a scalp brush.
Characteristics of the children in each category are shown in Box 2. The categories showed significant differences in age (P = 0.021) and class (P = 0.044, data not shown). When cases and carriers were combined and compared with uninfected children, they were significantly different by ethnicity (P = 0.010). Box 2 shows higher proportions of Sudanese, other African and Arabic children and lower proportions in other language groups in the cases and carriers groups. There was no difference by age, sex, class or braided hair (P = 0.060, 0.588, 0.093 and 0.343, respectively) between the cases/carriers and the uninfected children.
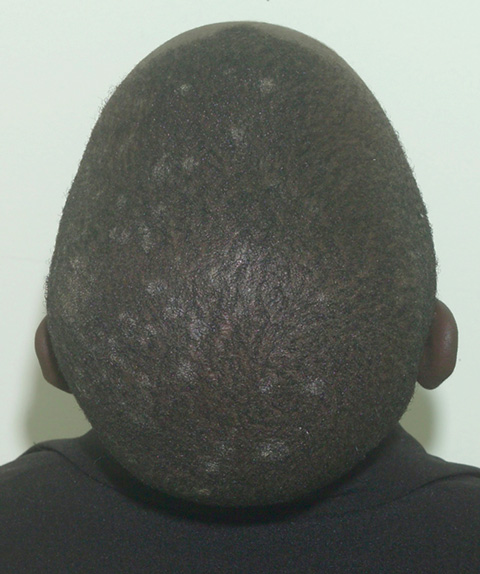
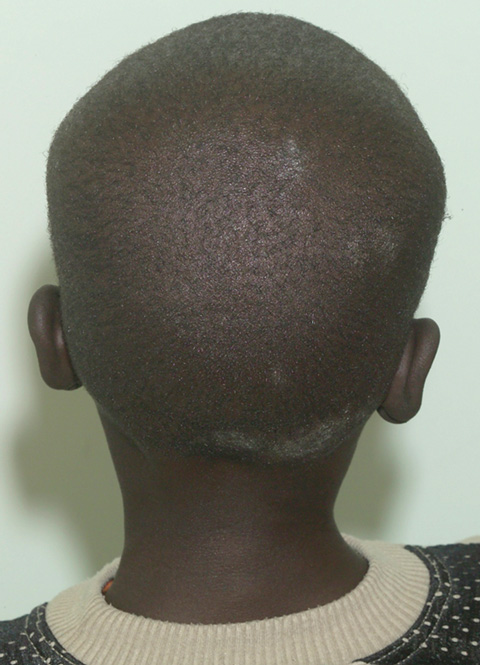
The clinical symptoms varied: 14 of the 23 cases had multiple discrete areas of scaling of the scalp, with minimal scalp inflammation (Box 3). Broken-off hairs and alopecia were not obvious, except in one child with a plaque of scaling, broken-off hairs and occipital lymphadenopathy (Box 4). Two children had diffuse dandruff-like scaling, and four had both tinea capitis and tinea corporis, one of whom also had tinea unguium. There was no correlation between the dermatophyte identified and the clinical pattern of disease.
Fifteen per cent of the children in our study were cases and 6% were asymptomatic carriers of dermatophytes. Whether this indicates an outbreak is difficult to determine. As tinea capitis is not notifiable in Victoria and there is no other routine collection of data, the number of expected cases is unknown. A similar study of anthropophilic tinea capitis in south-east London school children reported 2.5% as cases and 5.5% as carriers.3 In comparison, in south-western Ethiopia (where T. violaceum tinea capitis predominates), 16% of school children surveyed were infected, 13% were possibly infected and 17% were carriers.11 Other studies in Africa reported prevalences ranging from 5% in Nigerian primary school children12 to 59% in Ethiopian children.13 With the high proportion of African refugees in our group, our result of 15% cases and 6% carriers is within this range. It is probable that these African immigrant children arrived in Australia already infected with these dermatophytes, or acquired the infection from siblings, other family members or other African immigrants after their arrival.
The three dermatophyte species isolated in the study — T. violaceum, T. soudanense and M. audouinii — are uncommon in Australia. However, during the study we became aware of similar problems in other English-language schools in Melbourne, and a colleague reported that these dermatophytes were isolated from African immigrant children and their contacts in other jurisdictions in Australia. Similarly, in Victoria, we were aware of cases of T. soudanense and T. violaceum infections in children who had contact with African immigrant children. These findings closely parallel those reported from other countries.4,6,10
As the screening program took place near the end of the academic year and because many children were moving to other schools in the new academic year, follow-up was difficult. We therefore decided to treat all culture-positive children. This treatment may have been unnecessary for the carriers, but was considered appropriate during the investigation. Two letters were sent out to the parents of the affected children. One explained to the parents that their child had a positive result and that they should consult their doctor. The second letter was addressed to the doctor. It explained the reasons for the screening, the results and the recommended treatment: microfine griseofulvin at 15 mg/kg up to a maximum of 500 mg, orally, daily for 8 weeks. Children and their families were also encouraged to wash their hair with shampoo containing selenium sulphide (25 mg/mL; Selsun yellow; Abbott Australasia, Sydney, NSW) or ketoconazole (2%; Nizoral; Janssen-Cilag Australia, Sydney, NSW); the DHS provided shampoo to the school for distribution among the relevant families.
A common myth concerning the investigation of tinea capitis in school-age children is that the most likely source is contacts at school. However, American studies which investigated adults and siblings of infected patients showed a high prevalence of undiagnosed scalp infections and of carriers among household contacts.14-17 Women in these households were more likely to be asymptomatic carriers of anthropophilic dermatophytes.14-17 Therefore, contacts both at home and at school should be considered as possible carriers of fungi that cause tinea capitis, tinea corporis and tinea unguium.
Tinea capitis caused by anthropophilic dermatophytes can also mimic other scalp conditions, such as mild dandruff,18 so that the infection may pass unrecognised at the time of clinical examination. We found two children with diffuse dandruff-like scaling who were not clinically considered as infected, yet had positive laboratory results. Asymptomatic carriage of dermatophytes is well documented in populations where tinea capitis is endemic, at rates ranging from 5.5% to 24.5%.3,14 Follow-up studies of untreated asymptomatic carriers show that 7% to 21% will develop clinical tinea capitis, and that many (> 40%) remain asymptomatic carriers for 4 months or more.14,15
Determining what is appropriate treatment for carriers poses a problem. Regular shampooing with shampoo containing selenium sulphide (25 mg/mL) ketoconazole (2%), or 4% povidone-iodine (Betadine; Faulding, Sydney, NSW) has been shown to reduce the numbers of viable arthrospores on the hair and thus the likelihood of transmission.19 These shampoos produce good results after superficial contamination by contact with an infected person or with infected fomites. However, this is not an effective treatment for established infections in which the fungus has penetrated deep inside hair follicles or has infected the hairs. An alternative that has yet to be investigated is the use of pulse or intermittent oral therapy with an antifungal agent such as terbinafine or itraconazole, which concentrates in hard keratin, producing a depot effect.20
There were some limitations in our collection of samples for testing. The hair brush technique gave poor results from boys with closely cropped or shaved scalps, and from girls with tightly braided hair. Tinea capitis was confirmed in such children by microscopy and culture of hairs or scalp scrapings, but their brushings were either negative or produced only scant growth of dermatophytes. Toothbrushes can also be used to sample the scalp surface11,15 and would be the better choice for screening these children; scalp massager brushes give good results when used on hair that can be brushed. The potassium hydroxide test still has value, because treatment can be commenced within days of the specimen collection instead of after weeks of waiting for a culture result. Accordingly, plucking hair stubs or scraping skin scale from the scalp should also form part of any screening program.
Received 24 October 2007, accepted 21 January 2008
- Michelle E McPherson1,2
- Alan J Woodgyer3
- Kleete Simpson1
- Alvin H Chong4,5
- 1 Communicable Disease Control, Department of Human Services, Melbourne, VIC.
- 2 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
- 3 Microbiological Diagnostic Unit Public Health Laboratory, Department of Microbiology, University of Melbourne, Melbourne, VIC.
- 4 St Vincent’s Hospital Melbourne, Melbourne, VIC.
- 5 University of Melbourne, Melbourne, VIC.
We acknowledge the dermatological screening team who screened the children: Dr Janaka Akarawita, Dr Jenny Cahill, Dr Sarah Brennand, Dr Cara Holmes, Dr Hope Dinh, Dr Yan Pan. We also acknowledge Kristina Heinrich-Morrison and Pauline Lynch from the Department of Human Services and Caroline Davie from the Microbiological Diagnostic Unit, University of Melbourne. We would also like to thank the staff and children at the school.
None identified.
- 1. Hay RJ, Moore MK. Mycology. In: Burns T, Breathnach S, Cox N, Griffins C, eds. Rook’s textbook of dermatology. Vol 2. 7th ed. Oxford: Blackwell Science, 2004: 1405-1506.
- 2. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol 2000; 38: 255-287.
- 3. Hay RJ, Clayton YM, De Silva N, et al. Tinea capitis in south-east London — a new pattern of infection with public health implications. Br J Dermatol 1996; 135: 955-958.
- 4. Mounkassa B, Vandemeulebroucke E, Redlinsky S, et al. [Dermatophytes and tinea capitis in northern suburbs of Paris] [French]. J Mycol Med 2000; 10: 207-209.
- 5. Cuetara MS, Del Palacio A, Pereiro M, Noriega AR. Prevalence of undetected tinea capitis in a prospective school survey in Madrid: emergence of new causative fungi. Br J Dermatol 1998; 138: 658-660.
- 6. Heikkila H, Stubb S. Ringworm of the scalp among immigrants to Finland. Acta Derm Venereol 2004; 84: 333-334.
- 7. Maslen MM, Andrews PJ. Tinea due to Trichophyton violaceum in Victoria, Australia. Australas J Dermatol 1997; 38: 124-128.
- 8. Coloe SV, Baird RW. Dermatophyte infections in Melbourne: trends from 1961/64 to 1995/96. Pathology 1999; 31: 395-397.
- 9. Singh D, Patel DC, Rogers K, et al. Epidemiology of dermatophyte infection in Auckland, New Zealand. Australas J Dermatol 2003; 44: 263-266.
- 10. Lamb SR, Rademaker M. Tinea due to Trichophyton violaceum and Trichophyton soudanense in Hamilton, New Zealand. Australas J Dermatol 2001; 42: 260-263.
- 11. Figueroa JI, Hawranek T, Abraha A, Hay RJ. Tinea capitis in south-western Ethiopia: a study of risk factors for infection and carriage. Int J Dermatol 1997; 36: 661-666.
- 12. Nweze E. Etiology of dermatophytoses among children in northeastern Nigeria. Med Mycol 2001; 39: 181-184.
- 13. Woldeamanuel Y, Leekassa R, Chryssanthou E, et al. Prevalence of tinea capitis in Ethiopian school children. Mycoses 2005; 48: 137-141.
- 14. Ive FA. The carrier state of tinea capitis in Nigeria. Br J Dermatol 1966; 78: 219-221.
- 15. Pomeranz AJ, Sabnis SS, McGrath GJ, Esterly NB. Asymptomatic dermatophyte carriers in households of children with tinea capitis. Arch Pediatr Adolesc Med 1999; 153: 483-486.
- 16. Vargo K, Cohen BA. Prevalence of undetected tinea capitis in household members of children with disease. Pediatrics 1993; 92: 155-157.
- 17. Babel DE, Baughman SA. Evaluation of the adult carrier state in juvenile tinea capitis caused by Trichophyton tonsurans. J Am Acad Dermatol 1989; 21: 1209-1212.
- 18. Hay RJ. Endemic scalp ringworm: an object lesson in control of a common fungal infection. Curr Opin Infect Dis 2001; 14: 121-122.
- 19. Neil G, Hanslo D, Buccimazza S, Kibel M. Control of the carrier state of scalp dermatophytes. Pediatr Infect Dis J 1990; 9: 57-58.
- 20. Frieden IJ. Tinea capitis: asymptomatic carriage of infection. Pediatr Infect Dis J 1999; 18: 186-190.






Abstract
Objective: To investigate a reported increase in tinea capitis in an English-language school to determine if it was an outbreak and whether control measures were warranted.
Design: Cross-sectional study.
Setting and population: Primary school children enrolled at an English-language school in an outer suburb of Melbourne were screened for tinea capitis in November 2005 by clinical examination, collection of scalp, skin or nail specimens where clinically indicated, and scalp brushing.
Main outcome measures: Clinical diagnosis of tinea capitis confirmed by microscopy and culture.
Results: Parental consent was obtained for 180 children (98%), of whom 153 (85%) were screened. Dermatophytes were isolated from 21% (32/153) of the children screened, comprising 23 infected children (cases) and 9 carriers. Three dermatophyte species were identified: Trichophyton soudanense, Trichophyton violaceum and Microsporum audouinii. Cases and carriers were significantly different to non-cases by ethnicity (P = 0.010): a higher proportion came from Africa, notably Sudan, and Arabic countries.
Conclusions: Although our result may reflect what is expected in these migrant groups, tinea capitis caused by these three dermatophyte species is rare in Australian school children. Tinea capitis may continue to be a problem in these groups on account of continuing migration.