Clinical record
Abdominal computed tomography 1 month after presentation showed moderate ascites and a shrunken irregular liver contour, with a liver volume of 720 mL. Prominent vessels around the lesser curvature of the stomach suggested portal hypertension with collateral vessel formation. Doppler ultrasound examination a month later confirmed a small liver with coarse heterogeneous echotexture, and a macronodular surface. The portal vein was patent.
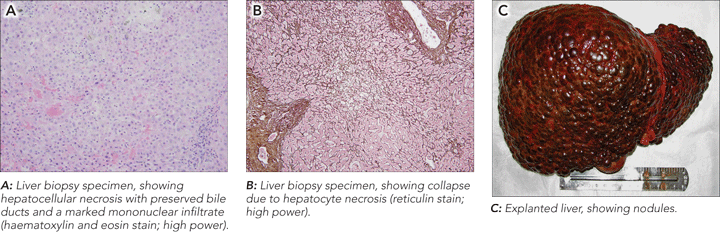
A liver biopsy 6 weeks after presentation showed massive hepatocellular necrosis with preserved bile ducts, collapsed parenchyma, and no recognisable residual hepatocytes (Figures, A and B). There were extensive mononuclear inflammatory infiltrates with few neutrophils. Perls staining was negative for iron.
A diagnosis was made of acute liver injury secondary to black cohosh ingestion. Over the subsequent weeks, the patient’s jaundice worsened, and her serum bilirubin level continued to rise. Frusemide was given to manage developing moderate ascites.
Sixty-two days after presentation, the patient developed asterixis (“hepatic flap”), indicating encephalopathy and liver failure. She was diagnosed with subfulminant liver failure suitable for liver transplantation and was listed urgently for transplant. Liver function tests showed a serum albumin concentration of 30 g/L and elevated serum concentrations of AST (202 U/L), ALT (73 U/L), ALP (191 U/L), GGT (64 U/L) and bilirubin (728 μmol/L). The INR was 1.8. Her renal function rapidly deteriorated, with serum creatinine concentration rising to 255 μmol/L. The MELD score reached 37.
A liver became available 5 days after the patient was listed, and a successful orthotopic liver transplantation was performed. Her postoperative course was uneventful.
The explanted liver weighed 744 g and was distorted by multiple nodules of varying size, from 5 mm to 50 mm (Figure, C). Microscopically, there were areas of extensive submassive necrosis, capsule distortion, collapse of the hepatic parenchyma, and mononuclear inflammatory infiltrates around the portal tracts. Cholestasis was prominent. Nodular regeneration with portal–portal linkage was also evident in some areas, as seen in the pretransplant biopsy.
Black cohosh is a herbal remedy used around the world for relief of menopausal symptoms. In Australia, over 200 listed medicines containing black cohosh are available without prescription.1 However, in the past decade, seven case reports of hepatotoxicity associated with black cohosh have been published.1-7
To our knowledge, our patient is the eighth reported case, and the sixth to require liver transplantation.Lessons from practice
Black cohosh is a herbal remedy used by millions of women worldwide for the relief of menopausal symptoms.
Emerging evidence of severe hepatotoxicity potentially linked with the use of black cohosh has raised concerns regarding its safety profile.
The community needs to be educated about potential risks of alternative and herbal medications such as black cohosh, and further regulations are required to monitor the safety of these preparations.
Black cohosh (Cimicifuga racemosa, also known as Actaea racemosa) is a perennial plant native to North America. The World Health Organization recognises its use for “treatment of climacteric symptoms such as hot flushes, profuse sweating, sleeping disorders and nervous irritability”.8 The American College of Obstetricians and Gynecologists stated that it may be helpful in the short term (6 months or less) for women with vasomotor symptoms of menopause.9 Although the exact mechanism of action is unknown, the primary active constituent of the black cohosh root is the terpene glycoside fraction, and the rhizome contains biologically active substances, including alkaloids, flavonoids, and tannins. However, despite the growing literature on the efficacy of black cohosh for menopausal symptoms, definite conclusions cannot be drawn because of the methodological shortcomings of available studies, such as lack of blinding, lack of long-term follow-up, and variations in product and dosage.10-12
Two safety reviews have found black cohosh extract to be well tolerated and adverse events to be rare when it is taken for up to 6 months.13,14 However, the seven case reports of hepatotoxicity potentially associated with black cohosh use in the past decade raise concern. Currently, there is no known biologically plausible mechanism to explain this hepatotoxicity, which is likely to be multifactorial. The plant contains both potentially hepatoprotective (triterpene glycosides) and hepatotoxic (salicylates, alkaloids) elements. Extracts and constituents of the rhizome have been shown to induce apoptosis and cell cycle arrest in human breast cancer cells,15,16 while extracts of the related plants Cimicifuga foetida and Cimicifuga dahurica inhibited proliferation of rat and mouse hepatocytes.17
The most likely cause of our patient’s liver failure was her use of black cohosh, although it has been recognised that 10% of patients receiving liver transplantation have idiopathic subfulminant liver failure.18 Our patient’s history of obesity treated with gastric bypass surgery 11 years before is unlikely to have contributed to the liver failure as she had shown no abnormalities of liver function previously, and no features of steatohepatitis were seen in the pretransplant liver biopsy specimens. Our case is also notable as the patient had used black cohosh intermittently at half the manufacturer’s recommended dose for 3 years, and at the manufacturer’s recommended dose (20 mg twice a day) for only 2 months before symptom onset.
There is a widespread belief in the community that “natural” plant substances are safe, effective and free of side effects. Various regulatory bodies now recognise the association between black cohosh use and hepatotoxicity, and many recommend warning labels. The Therapeutic Goods Administration (TGA) was the first in the world to announce, in February 2006, that medicines containing black cohosh must include the label: “Warning: black cohosh may harm the liver in some individuals. Use under the supervision of a healthcare professional”.19 In November 2007, the TGA revised the warning to: “In very rare cases, black cohosh has been associated with liver failure. If you experience yellowing of the skin or eyes, dark urine, nausea, vomiting, unusual tiredness, weakness, stomach or abdominal pain, and/or loss of appetite, stop using this product and see your doctor”.20
- 1. Adverse Drug Reactions Advisory Committee. Hepatotoxicity with black cohosh. Aust Adv Drug Reactions Bull 2006; 25 (2). http://www.tga.gov.au/adr/aadrb/aadr0604.htm#a1 (accessed Feb 2008).
- 2. Cohen SM, O'Connor AM, Hart J, et al. Autoimmune hepatitis associated with the use of black cohosh: a case study. Menopause 2004; 11: 575-577.
- 3. Levitsky J, Alli TA, Wisecarver J, et al. Fulminant liver failure associated with the use of black cohosh. Dig Dis Sci 2005; 50: 538-539.
- 4. Lontos S, Jones RM, Angus PW, Gow PJ. Acute liver failure associated with the use of herbal preparations containing black cohosh. Med J Aust 2003; 179: 390-391.
- 5. Lynch CR, Folkers ME, Hutson WR. Fulminant hepatic failure associated with the use of black cohosh: a case report. Liver Transpl 2006; 12: 989-992.
- 6. Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust 2002; 177: 440-443. <MJA full text>
- 7. Dunbar K, Solga SF. Black cohosh, safety, and public awareness. Liver Int 2007; 27: 1017.
- 8. World Health Organization. WHO monographs on selected medicinal plants. Vol 2. Geneva: WHO, 2002: 55-65.
- 9. American College of Obstetricians and Gynecologists Committee on Practice Bulletins — Gynecology. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Use of botanicals for management of menopausal symptoms. Obstet Gynecol 2001; 97 (6 Suppl): 1-11.
- 10. Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 2002; 137: 805-813.
- 11. Osmers R, Friede M, Liske E, et al. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol 2005; 105: 1074-1083.
- 12. Pockaj BA, Gallagher JG, Loprinzi CL, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol 2006; 24: 2836-2841.
- 13. Dog TL, Powell KL, Weisman SM. Critical evaluation of the safety of Cimicifuga racemosa in menopause symptom relief. Menopause 2003; 10: 299-313.
- 14. Huntley A. The safety of black cohosh (Actaea racemosa, Cimicifuga racemosa). Expert Opin Drug Saf 2004; 3: 615-623.
- 15. Einbond LS, Shimizu M, Xiao D, et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast Cancer Res Treat 2004; 83: 221-231.
- 16. Hostanska K, Nisslein T, Freudenstein J, et al. Cimicifuga racemosa extract inhibits proliferation of estrogen receptor-positive and negative human breast carcinoma cell lines by induction of apoptosis. Breast Cancer Res Treat 2004; 84: 151-160.
- 17. Tian Z, Yang M, Huang F, et al. Cytotoxicity of three cycloartane triterpenoids from Cimicifuga dahurica. Cancer Lett 2005; 226: 65-75.
- 18. Lynch SV, Balderson GA, editors. ANZLT Registry report 2004. Brisbane: Australia and New Zealand Liver Transplantation Registry, 2004.
- 19. Adverse Drug Reactions Advisory Committee and Australian Government Therapeutic Goods Administration. Black cohosh and liver toxicity — an update. Aust Adverse Drug React Bull 2007; 26 (3). http://www.tga.gov.au/adr/aadrb/aadr0706.htm (accessed Feb 2008).
- 20. Australian Government Department of Health and Ageing, Therapeutic Goods Administration. Required advisory statements for medicine labels. Proposed update 3.1. Consultation document. November 2007. http://www.tga.gov.au/meds/drrasmlupd3-1.pdf (accessed Feb 2008).





None identified.