Human papillomaviruses (HPVs), spread through genital contact, are the major cause of cervical cancer. Two HPV vaccines, the bivalent Cervarix (GlaxoSmithKline) and the quadrivalent Gardasil (Merck), are available. Both are designed to prevent HPV types 16 and 18 infections, which, worldwide, are responsible for about 70% of cervical cancer cases, 50% of high-grade precancerous lesions and 25% of low-grade lesions.1 Gardasil is designed to also prevent HPV types 6 and 11 infections, associated with most genital warts2 and around 8%–10% of low-grade cervical lesions.3 Gardasil was licensed by the Australian Therapeutic Goods Administration in June 2006 for use in women and girls aged 9–25 years and boys aged 10–15 years. Cervarix was licensed more recently for use in females aged 10–45 years. It is likely that Gardasil will also be approved for older women in the near future.
Administering the vaccines to the pre-adolescent population maximises the chances of most of the population achieving immunity before HPV exposure. As a more robust immune response to vaccination is achieved at this age, protection is likely to endure through the years of maximal exposure. However, extended follow-up of populations in clinical trials will help to determine whether a booster is required. Favourable estimates of the cost-effectiveness of a catch-up immunisation program for women up to the age of 26 years in economic models adapted to Australian data4,5 led to federal government funding of a universal immunisation program for girls aged 12 and 13 in Australia, with a 2-year catch-up program for older adolescent and young adult women. This program has commenced through schools and general practitioner clinics.
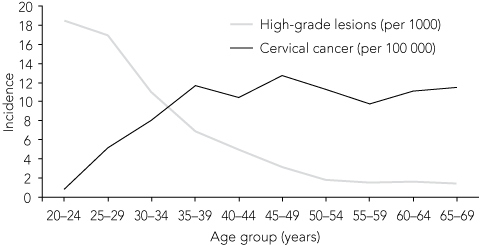
The role of these vaccines for women older than 26 years is yet to be established. However, demand is likely to be strong, given (1) that high-grade dysplasia from HPV acquired several years earlier still occurs in women well over this age (Box 1); (2) that cervical cancer incidence peaks in women aged over 40 years (mainly from infection 15 or more years earlier); (3) the enthusiastic participation of women in this age group in clinical trials of HPV vaccines; and (4) informal feedback from educational sessions. The most important issues include: whether an older woman newly infected with HPV has a risk of cervical cancer similar to that of a younger woman; whether the vaccine provides protection against new infection at this age; whether new infections occur in older age; and whether the vaccine has a role in preventing reactivation of latent infection. The data to answer these questions conclusively are not yet available.
Molecular epidemiological studies have conclusively established the causal association between so-called high-risk HPV genotypes and cervical cancer.7
Acquisition of HPV infection of the genital tract occurs rapidly after sexual debut. One study showed a cumulative incidence of HPV of about 40% in women after sexual debut or after a new sexual partner, over a 24-month follow-up.8 About 10% and 4% of these infections were HPV 16 and 18, respectively. However, most infections, including those with high-risk types, do not lead to dysplasia and will clear spontaneously within 2 years, leaving no residual detectable HPV DNA.9 Women who do develop cytologically or histologically detectable cervical lesions in response to HPV infection will eventually mount an effective cell-mediated immune response, which causes lesion regression.10 In a small proportion of women, persistent infection continues; this is linked to the development of high-grade cervical lesions and cervical cancer.11
A measurable systemic immune response, with neutralising HPV type-specific antibodies, occurs in only 50%–60% of women infected with HPV. This antibody response is weak and slow, taking many months from initial infection.12 Natural history studies provide conflicting evidence for the protection afforded by naturally acquired antibodies against reinfection with the same genotype,13 although there is evidence of protection at the cellular level, which may be relevant even in the absence of antibodies.14 In some hosts, the virus can remain for many years in a latent state in basal epithelial cells at levels that can only be detected with highly sensitive detection methods in biopsy material, but might become reactivated with suppression or senescence of immunity.10
Burden of disease due to HPV cervical infectionGlobally, the burden of disease due to cervical cancer is enormous. The Asian region contributes the most in absolute numbers (incidence, 265 884 per year),15 while Australia accounts for about 800 new cases and 300 deaths per year.16
As high-grade dysplasia, the precursor lesion to cancer,11 precedes cancer development by up to 15 years, there is opportunity for secondary prevention. However, owing to the complexity and cost of implementing a population-based screening program, only about 5% of women in developing countries are screened, compared with up to 70% of women in developed countries.17 Further, Pap test sensitivity is dependent on the expertise of laboratories. Even in countries with high-quality programs with good participation in regular screening, 25% of squamous cell carcinomas still occur in adequately screened women.6 In addition, Pap test screening has not reduced the incidence of adenocarcinoma. In Australia, the lifetime risk of cervical cancer is estimated to be 2.4% without Pap test screening and 0.77% for women who are screened at an average rate.6 Since HPV genital infection is so common, it is not surprising that screening comes with a 34% lifetime risk of an abnormal Pap test.
Estimates of the prevalence of HPV infection among women range from 2% to 44%, depending on age.9,18 In an Australian study of genital HPV prevalence, interim analyses showed that 24% of women attending clinics for Pap test screening show high-risk HPV DNA.19 HPV 16 and 18 were the two most common high-risk genotypes, as reported in most other studies.20 Age stratification of HPV prevalence showed the highest rates in women under 25 years of age, a decrease from 30 years of age and a second smaller peak in those older than 45 years.20
The second lifetime peak in HPV prevalence has been found in many population studies, although it is most pronounced in developing countries (Box 2).20,21 While most prevalent HPV infections acquired at a younger age are cleared, in older women prevalent infection is more likely to represent prolonged persistence.22 Prolonged persistent infection places the woman at higher risk of progression to cancer. The second peak in HPV prevalence raises additional questions, such as whether this is reactivation of latent infection acquired earlier in life, acquisition of a new infection with changes in sexual behaviour, or a reduced ability to respond to new or previously encountered infectious agents as a result of decline in immune function.23 The gradual loss of type-specific antibodies to HPV12,23 and altered immunological function in older women with persistent HPV infection may also play a role.24 In addition, a cohort effect, related to secular changes in sexual behaviour or circulation of the virus over time cannot be excluded.9
Prospective studies show decreasing, although continuing, acquisition of HPV infections throughout the lifespan; the annual incidence is 5%–10% in women aged 25–80 years of age.25-27 Some studies have suggested a second peak in incidence in later life.22,28 In addition, we know that sexual activity with new partners continues with age, with 17% of men and 11% of women aged 35–44 years in the United Kingdom reporting new partners in the previous year.29 In Australia, 12% of men and 6% of women aged 30–39 years report more than one partner in the previous year.30 While a woman with a high number of past partners may have a higher likelihood of past exposure, this same woman is more likely to have more sexual partners in the future than a woman of the same age with few past sexual partners.31 It is possible that this type of heterogeneity in sexual behaviour may balance out the potential benefit of a vaccine in these two groups of women. In addition, concurrent partnerships are not uncommon,32 and awareness of whether one’s partner has other partners is not always accurate.32
The presence of antibodies to specific HPV types in serum is considered a marker of past infection, although it is an underestimate of true exposure. Most surveys show an increase in the proportion of the population seropositive to specific HPV types until mid adulthood, and then a plateau or reduction.33,34 For HPV 16, this proportion was as high as 25% in a representative sample of United States women aged 20–49 years.34 Concomitant HPV 16 and 18 seropositivity is generally low across all age groups; in pregnant Finnish women, concomitant HPV 16 and 18 seropositivity was 4.4% in the age group 14–22 years, and 6.2% in the age group 23–31 years.33 Increasing numbers of sexual partners have been correlated with increasing HPV seropositivity: about 25%–30% of women with four or more lifetime sexual partners have shown evidence of HPV 16 antibodies,34,35 with a slightly lower percentage for HPV 18.
Phase III clinical trials of the bivalent and quadrivalent vaccines, involving around 40 000 young women from the Asia–Pacific region, South and North America and Europe, also provide important epidemiological data on previous HPV exposure.36-38 In the quadrivalent vaccine trials, women were aged 16–26 years with a maximum of four sexual partners and no history of abnormal Pap tests. In the bivalent vaccine trials, women were aged 15–25 years, with a maximum of six sexual partners; women with a history of low-grade abnormal Pap tests were included. Baseline analyses of these studies have shown that up to 30% of women had evidence of either prior or current infection with any one of four of the vaccine-type HPVs (as detected by HPV DNA status or serology). However, most of this subgroup had been exposed to only one type: only about 7% had been exposed to both HPV 16 and HPV 18.37
A combined analysis of Phase II and Phase III clinical trials assessed the efficacy of quadrivalent vaccine in preventing HPV 16- or 18-related cervical intraepithelial neoplasia (CIN) grade 2 or 3 or adenocarcinoma in situ. This included the Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I study, in which Australian women participated.38 In women without the vaccine type of HPV infection at baseline, after a mean of 3 years of follow-up (according to the protocol and modified intention-to-treat analysis), efficacy was 98%–99% (95% CI, 93%–100%).36 Efficacy in the entire population for any cervical lesions regardless of causal HPV type was 18%. In relation to the latter estimate, many women already had a low-grade lesion on cytology or evidence of HPV 16 or 18 infection at baseline. It is expected that efficacy will increase as prevalent infection and disease are cleared and new infections prevented, and better reflect the effectiveness of this vaccine in a real population over time.
Phase II and Phase III clinical trials of the bivalent HPV vaccine have shown 100% efficacy against CIN grades 1 to 3 due to HPV 16 or 18 in women previously unexposed to these vaccine types in an according-to-protocol analysis of data up to 4.5 years,39 and 90% to 100% efficacy against CIN grade 2 or 3 due to HPV 16 and/or 18 in intention-to-treat and post-hoc analyses after 15 months of follow-up. The latter study is the single largest HPV vaccine efficacy trial, in which Australian women are also participating.37
In an immunogenicity study of the bivalent vaccine, 100% of women up to the age of 55 years seroconverted to both HPV types. While levels of antibodies were lower than in the younger age group, they were still at least 10-fold higher than those caused by natural infection.40 This vaccine was also well tolerated in older women, who had a lower incidence of local reactions than the younger age group.
Women who had evidence of past exposure to HPV 16 or 18 at the time of vaccination were afforded protection against the other vaccine-preventable HPV subtypes. For women who had evidence of naturally acquired antibodies to HPV 16 or 18, but no HPV DNA (indicating cleared past infection), the natural antibody was boosted, which might have provided benefits against infection in the longer term.41 Women who were currently infected with either HPV 16 or 18 (positive HPV DNA) were not afforded any protection against high-grade lesions due to the relevant HPV type. Vaccination of women with cervical dysplasia did not worsen or promote regression of existing cervical lesions.
While it is impossible for a GP to give women older than 26 years an exact assessment of their potential for benefit, women can be provided with information to make a balanced decision about the costs and benefits of vaccination (Box 3).
1 Incidence of HSIL per 1000 women screened and incidence of cervical cancer* per 100 000 population, Australia, 20036
 | |||||||||||||||
2 Age-specific prevalence of human papillomavirus (HPV) among women with normal cytology results (n = 157 879), from a systematic review and meta-analysis of 78 studies
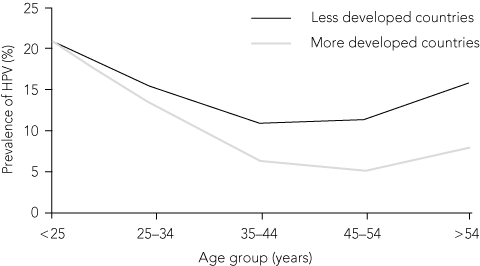 | |||||||||||||||
|
Originally published in Lancet Infect Dis.20 Copyright Elsevier, 2007. | |||||||||||||||
3 Clinical questions
Are human papillomavirus (HPV) vaccines appropriate for sexually experienced women? Even with increasing age and numbers of sexual partners, most women do not have evidence of past exposure to HPV types 16 or 18. Older women have robust immune responses to the bivalent HPV vaccine, and so should derive benefit from the vaccine if exposed to HPV 16 or 18 in the future.
Is it too late to vaccinate a woman if she has a history of HPV disease shown by clinical evidence such as an abnormal Pap test or genital warts? Evidence to date indicates that vaccination will have no effect on current or prevalent disease due to any HPV type. However, vaccination would ensure protection from future infection with oncogenic HPV types covered by the vaccine.
Can a woman’s ongoing risk of acquiring HPV be determined? Future risk of exposure is difficult to determine accurately on the basis of past and current sexual history. This is because of patterns and changes in sexual behaviour through life, the potential for transmission through successive monogamous relationships, and inaccuracies in predictions about concurrency of sexual partners.
Should there be an age cut-off for vaccination? As a woman ages, natural immunity to HPV wanes, but also the incidence of new HPV infection decreases and the time to develop cancerous lesions from HPV must be balanced against the likelihood of other age-related diseases. Currently, the bivalent vaccine is licensed for use in women up to 45 years.
Should prevaccination HPV DNA genotype-specific testing or serology be undertaken? No. Currently there are no validated, approved and readily available HPV type-specific polymerase chain reaction or serological assays. Were they available and used, the process would add considerable expense to an already expensive intervention.
- S Rachel Skinner1,2
- Suzanne M Garland3,4
- Margaret A Stanley5
- Marian Pitts6
- Michael A Quinn4,7
- 1 School of Paediatrics and Child Health, University of Western Australia, Perth, WA.
- 2 Vaccine Trials Group, Telethon Institute for Child Health Research, Perth, WA.
- 3 Department of Microbiology and Infectious Diseases, Royal Women’s Hospital, Melbourne, VIC.
- 4 Department of Obstetrics and Gynaecology, University of Melbourne, Melbourne, VIC.
- 5 Department of Pathology, University of Cambridge, Cambridge, UK.
- 6 Australian Research Centre In Sex, Health and Society, La Trobe University, Melbourne, VIC.
- 7 Oncology and Dysplasia Unit, Royal Women’s Hospital, Melbourne, VIC.
Rachel Skinner has received honoraria for attending GlaxoSmithKline (GSK) Cervical Cancer Working Party meetings and CSL Gardasil Advisory Board meetings. GSK and CSL have paid for travel costs to attend international HPV conferences. She has received speaker fees from GSK. Suzanne Garland has been an adviser to Merck and has had her costs for presenting at meetings covered by those funding the research. She is a member of both the CSL and GlaxoSmithKline advisory boards for HPV vaccines. Margaret Stanley is a paid consultant for Merck Vaccines (West Point, Pa, USA) and GSK Biologicals (Rixensart, Belgium). She has received speaker fees and travel assistance from Merck and GSK. Marian Pitts has received funding from GSK to attend an international symposium and is a member of the GSK Cervical Cancer Prevention Working Group. Michael Quinn is the chair of the GSK Cervix Cancer Prevention Advisory Board, for which he is paid an honorarium for attending meetings.
- 1. Clifford G, Franceschi S, Diaz M, et al. HPV type-distribution in women with and without cervical neoplastic disease. Vaccine 2006; 24 Suppl 3: S26-S34.
- 2. Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis 2005; 192: 2099-2107.
- 3. Clifford GM, Rana RR, Franceschi S, et al. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 1157-1164.
- 4. Regan DG, Philp DJ, Hocking JS, Law MJ. Modelling the impact of a vaccine on HPV 16 transmission in Australia. Sex Health 2007; 4: 147-163.
- 5. Kulasingam S, Connelly L, Conway E, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex Health 2007; 4: 165-175.
- 6. Australian Institute of Health and Welfare. Cervical screening in Australia 2004–2005. Canberra: AIHW, 2007. http://www.aihw.gov.au/publications/index.cfm/title/10457 (accessed Jan 2008).
- 7. Bosch F, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55: 244-265.
- 8. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157: 218-226.
- 9. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24 Suppl 1: S1-S15.
- 10. Stanley M. Immune responses to human papillomavirus. Vaccine 2006; 24 Suppl 1: S16-S22.
- 11. Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005; 337: 76-84.
- 12. Ho GY, Studentsov Y, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev 2004; 13: 110-116.
- 13. Viscidi RP, Schiffman M, Hildesheim A, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev 2004; 13: 324-327.
- 14. De Jong A, van Poelgeest MI, van der Hulst JM, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res 2004; 64: 5449-5555.
- 15. International Agency for Research on Cancer. Globocan 2002 [database]. Lyon: IARC, 2005. http://www-dep.iarc.fr (accessed Jan 2008).
- 16. Australian Institute of Health and Welfare, Australasian Association of Cancer Registries. Cancer in Australia 2001. AIHW cancer series no. 28. Canberra: AIHW, 2004.
- 17. Garland S. Can we really beat cervical cancer? Med J Aust 2003; 178: 647-650. <MJA full text>
- 18. Bosch FX, de Sanjosé S. Chapter 1. Human papillomavirus and cervical cancer — burden and assessment of causality. J Natl Cancer Inst Monogr 2003; (31): 3-13.
- 19. Garland SM, Brotherton J, Condon J, et al. Human papillomavirus genotype prevalence in Australian women (Indigenous, non-Indigenous, urban, rural populations). International Papillomavirus Conference; 2007 Nov 2–9; Beijing, China.
- 20. de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7: 453-459.
- 21. Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer 2006; 119: 2677-2684.
- 22. Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 2005; 191: 1808-1816.
- 23. Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, age and immune function: toward a lifespan approach. J Behav Med 2006; 29: 389-400.
- 24. García-Piñeres AJ, Hildesheim A, Herrero R, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res 2006; 66: 11070-11076.
- 25. Munoz N, Mendez F, Posso H, et al. Incidence, duration and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 2004; 190: 2077-2087.
- 26. Sellors J, Karwalajtys TL, Kaczorowshi J, et al; Survey of HPV in Ontario Women Group. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ 2003; 168: 421-425.
- 27. Franco E, Villa LL, Sobrinho JP, et al. Epidemiology of acquistion and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180: 1415-1423.
- 28. Grainger MJ, Seth R, Guo L, et al. Cervical human papillomavirus screening among older women. Emerg Infect Dis 2005; 11: 1680-1685.
- 29. Johnson A, Mercer CH, Erens B, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet 2001; 358: 1835-1842.
- 30. de Visser RO, Smith AM, Rissel CE, et al. Sex in Australia: heterosexual experience and recent heterosexual encounters among a representative sample of Australian adults. Aust N Z J Public Health 2003; 27: 146-154.
- 31. Burchell AN, Winer RL, de Sanjosé S, Franco EL. Epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006; 24 Suppl 3: S52-S61.
- 32. Drumright LN, Gorbach PM, Holmes KK. Do people really know their sex partners? Concurrency, knowledge of partner behavior, and sexually transmitted infections within partnerships. Sex Transm Dis 2004; 31: 437-442.
- 33. Lehtinen M, Kaasila M, Pasanen K, et al. Seroprevalence atlas of infections with oncogenic and non-oncogenic human papillomaviruses in Finland in the 1980s and 1990s. Int J Cancer 2006; 119: 2612-2619.
- 34. Stone KM, Karem KL, Sternberg MR, et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186: 1396-1402.
- 35. Wang S, Schiffman M, Shields TB, et al. Seroprevalence of human papillomavirus- 16, -18, -31, and -45 in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer 2003; 80: 1248-1254.
- 36. Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369: 1861-1868.
- 37. Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161-2170.
- 38. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356: 1928-1943.
- 39. Harper DM, Franco EL, Wheeler C, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow up from a randomised controlled trial. Lancet 2006; 367: 1247-1255.
- 40. Schwarz TF, Dubin GO; HPV Vaccine Study Investigators for Adult Women. An AS04-containing human papillomavirus (HPV) 16/18 vaccine for prevention of cervical cancer is immunogenic and well-tolerated in women 15-55 years old. American Society of Clinical Oncology Annual Meeting; 2006 Jun 2–6; Atlanta, Ga. Abstract 1008. http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=34377 (accessed Jan 2008).
- 41. Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006; 24: 5571-5583.





Abstract
Human papillomaviruses (HPVs) are the major cause of cervical cancer. Cervical cancer mortality has been reduced in Australia because of effective screening programs, but there are still about 800 new cases and 300 deaths per year. Worldwide, mortality and morbidity are high.
Australia was the first country to introduce fully funded immunisation with a quadrivalent HPV vaccine for girls aged 12 and 13 in schools. A 2-year catch-up program covers all women to the age of 26 years.
Age stratification of HPV prevalence showed the highest rates in women under 25 years of age, a decrease in women from 30 years of age and a second smaller peak in those over 45 years.
Recently, a bivalent HPV vaccine has been licensed for use in women aged up to 45 years. Older women have robust immune responses to the bivalent HPV vaccine, and so should derive benefit from the vaccine if exposed to HPV type 16 or 18 in the future. It is likely that this vaccine will need to be purchased by women in the older age group (27–45 years).