Takotsubo cardiomyopathy is an increasingly recognised syndrome characterised by transient apical left ventricular dysfunction in the absence of significant coronary artery disease. We describe a case of Takotsubo cardiomyopathy complicated by Dressler’s syndrome. To our knowledge, these two conditions have not previously been reported in combination.
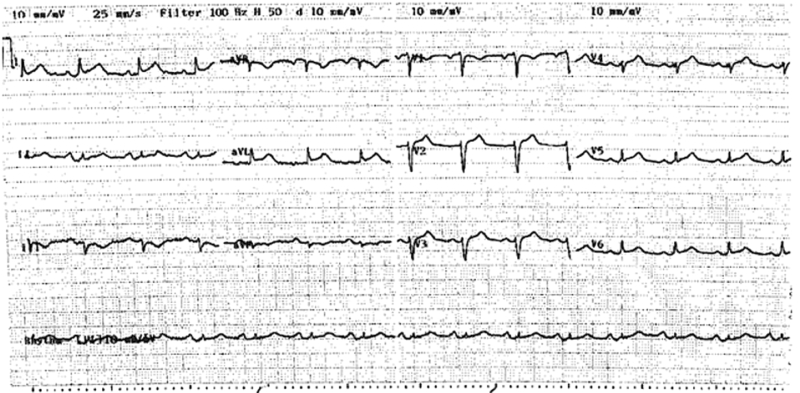
A 75-year-old woman presented with acute onset of discomfort in the chest, left scapula, neck and arm after being informed of the unexpected death of her son, and 2 days after undergoing an uncomplicated laparoscopic cholecystectomy. Her medical history included Graves’ disease and a hysterectomy. She had no modifiable risk factors for coronary artery disease. Examination revealed a heart rate of 90 beats per minute and a blood pressure of 120/80 mmHg, with no evidence of acute pulmonary oedema. At presentation, an electrocardiogram demonstrated 1–2 mm ST elevation in leads V1–V6, I and aVL (Box 1).
The patient was immediately transferred to the cardiac catheter laboratory, where angiography revealed angiographically normal coronary arteries but extensive anteroapical and inferoapical ballooning consistent with Takotsubo cardiomyopathy.1-9 Peak creatinine kinase and troponin I levels were 245 μg/L (reference range [RR], < 200 μg/L) and 6.62 μg/L (RR, < 0.1 μg/L), respectively. The patient’s in-hospital course was complicated by heart failure, necessitating treatment with intravenous diuretics. The patient also developed pleuritic-type chest pain, but computed tomographic pulmonary angiography (CTPA) excluded pulmonary embolism. The patient was discharged 3 days after admission on a medication schedule of metoprolol 25 mg twice a day, and perindopril 2.5 mg, frusemide 40 mg and aspirin 150 mg once a day.
A follow-up echocardiogram 6 weeks later showed normal left ventricular function and size, with a calculated ejection fraction of 72%. A trivial pericardial effusion measuring less than 0.2 cm was noted. All cardiac medications were ceased at that point.
About a week after the follow-up echocardiogram, the patient presented again with severe pleuritic chest pain that was relieved by sitting forward. The pain was associated with low-grade fever, shortness of breath and a dry cough. She did not report any other symptoms suggestive of an upper respiratory tract infection. Examination revealed signs of a pericardial friction rub and bilateral pleural effusions but no evidence of cardiac tamponade.
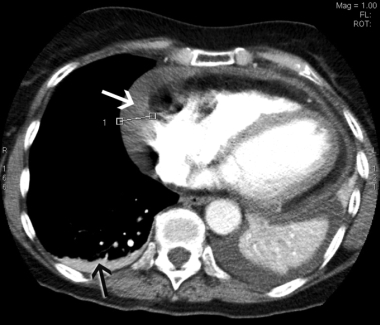
In view of the recent cholecystectomy, the patient underwent repeat CTPA, which excluded a pulmonary embolus but revealed a moderate pericardial effusion measuring up to 2.1 cm laterally and small bilateral pleural effusions (Box 2). An echocardiogram confirmed the pericardial effusion but showed no evidence of cardiac tamponade. Inflammatory markers were markedly elevated, with a C-reactive protein level of 290 mg/L (RR, < 12 mg/L) and an erythrocyte sedimentation rate of 103 mm/h (RR, < 21 mm/h). The patient had low-grade anaemia (haemoglobin level, 96 g/L; RR, 115–165 g/L), but this improved to 116 g/L without treatment. Her white cell count was elevated at 16 × 109/L (RR, 4–11 × 109/L), with predominant neutrophilia, and her platelet count was significantly elevated at 800 × 109/L (RR, 150–400 × 109/L). Renal function, urinalysis, autoimmune markers and a repeat troponin I measurement were normal. These findings were consistent with a diagnosis of Dressler’s syndrome.
Treatment with ibuprofen 400 mg three times daily resulted in a rapid reduction in pain and levels of inflammatory markers. Inflammatory marker levels normalised within 3 months.
Takotsubo cardiomyopathy was first described in Japan in 1991. It is characterised by transient apical left ventricular ballooning in the absence of significant coronary artery disease.1
Our patient’s initial presentation was consistent with a diagnosis of Takotsubo cardiomyopathy. The pathogenesis of this condition is not well understood but is postulated to be caused by coronary artery vasospasm in association with intense emotional or physical stress, leading to apical myocardial stunning.2-4 As a result there is often minimal myocardial necrosis, as reflected by the minor cardiac enzyme rise in this case.5 Left ventricular function usually returns to normal within 1–4 weeks, as seen in our patient.6-8 The role of catecholamines in the pathogenesis of Takotsubo cardiomyopathy has been documented in numerous studies.9-12
Dressler’s syndrome was first described by Dressler in 1956.13 It is characterised by a late-presentation pericarditis, presenting weeks to months after a myocardial infarction. Six weeks after the diagnosis of Takotsubo cardiomyopathy, our patient displayed typical features of Dressler’s syndrome: pleuritic chest pain, pericardial friction rub with associated effusion, fever, leukocytosis, pleural effusions and elevated levels of inflammatory markers.13
The pathogenesis of Dressler’s syndrome is thought to be immune-mediated, as evidenced by late onset of the syndrome. The putative pathogenetic sequence begins with myocardial injury that releases cardiac antigens and stimulates antibody formation. The immune complexes that are generated then deposit onto the pericardium, pleura and lungs, eliciting an inflammatory response.14-16
Earlier studies quoted the incidence of Dressler’s syndrome to be as high as 3.3%–4.8% after myocardial infarction,17-18 but more recent estimates have been significantly lower, possibly as a result of reperfusion strategies that limit the size of the infarct and the release of cardiac antigens that stimulate an immune response.19
Our patient presented with Takotsubo cardiomyopathy and developed Dressler’s syndrome 6 weeks later. To our knowledge, Dressler’s syndrome following Takotsubo cardiomyopathy has not been previously described in the literature. This may be because the extent of biochemical myocardial damage demonstrated is often small in Takotsubo cardiomyopathy, whereas Dressler’s syndrome is often associated with significant myocardial necrosis. For this reason, the diagnosis of Dressler’s syndrome in our patient was unexpected and the mechanism is unclear.
- 1. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141: 858-865.
- 2. Ako J, Sudhir K, Farouque HM, et al. Transient left ventricular dysfunction under severe stress: brain–heart relationship revisited. Am J Med 2006; 119: 10-17.
- 3. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006; 27: 1523-1529.
- 4. Kurisu S, Sato H, Kawagoe T, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002; 143: 448-455.
- 5. Dec GW. Recognition of the apical ballooning syndrome in the United States. Circulation 2005; 111: 388-390.
- 6. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005; 111: 472-479.
- 7. Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart 2003; 89: 1027-1031.
- 8. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris–Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001; 38: 11-18.
- 9. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539-548.
- 10. Ito K, Sugihara H, Katoh S, et al. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT — comparison with acute coronary syndrome. Ann Nucl Med 2003; 17: 115-122.
- 11. Akashi YJ, Nakazawa K, Sakakibara M, et al. 123I-MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004; 45: 1121-1127.
- 12. Kurisu S, Inoue I, Kawagoe T, et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J 2004; 68: 77-81.
- 13. Dressler W. A post-myocardial infarction syndrome: preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis. J Am Med Assoc 1956; 160: 1379-1383.
- 14. Khan AH. The postcardiac injury syndromes. Clin Cardiol 1992; 15: 67-72.
- 15. De Scheerder I, De Buyzere M, Robbrecht J, et al. Postoperative immunologic response against contractile proteins after coronary bypass surgery. Br Heart J 1986; 56: 440-444.
- 16. Kennedy HL, Das SK. Postmyocardial infarction (Dressler’s) syndrome: report of a case with immunological and viral studies. Am Heart J 1976; 91: 233-239.
- 17. Welin L, Vedin A, Wilhelmsson C. Characteristics, prevalence, and prognosis of postmyocardial infarction syndrome. Br Heart J 1983; 50: 140-145.
- 18. Lichstein E, Arsura E, Hollander G, et al. Current incidence of postmyocardial infarction (Dressler’s) syndrome. Am J Cardiol 1982; 50: 1269-1271.
- 19. Shahar A, Hod H, Barabash GM, et al. Disappearance of a syndrome: Dressler’s syndrome in the era of thrombolysis. Cardiology 1994; 85: 255-258.





None identified.