Autoimmune hepatitis is a rare but increasingly recognised serious complication of treatment with the tumour necrosis factor-α (TNF-α) blocking agent, infliximab. This report adds to the small but growing number of articles describing this significant adverse effect. Baseline liver function should be routinely tested in all patients receiving anti-TNF-α agents, and periodic monitoring for the development of hepatitis is important.
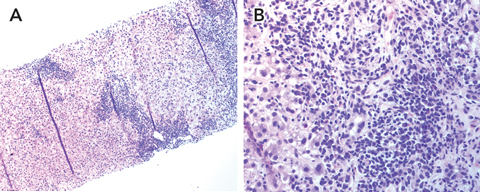
Results of her blood tests at cessation of infliximab therapy are shown in Box 1. Computed tomography of the abdomen showed diffusely non-homogeneous liver texture consistent with chronic liver disease, with no intra- or extrahepatic duct dilatation. A past cholecystectomy was also evident. A liver biopsy showed marked inflammatory infiltrate, particularly around the portal tract, with neutrophils, lymphoid, and plasma cells, bridging necrosis and piecemeal necrosis consistent with autoimmune chronic active hepatitis (Box 2).
Minor side effects of treatment with anti-TNF-α agents are common, and include upper respiratory tract infections, rash, myalgias, asthenia, sinusitis, flushing, fever and headache.1 More serious side effects include the reactivation of latent Mycobacterium tuberculosis; reactivation of hepatitis B in chronic carriers; infusion reactions (related to induction of anti-chimeric antibodies against infliximab); severe neutropenia and thrombocytopenia; demyelinating disorders; autoimmune antibody formation; and hepatotoxicity.
Minor abnormalities in LFT results are relatively common with the use of anti-TNF-α agents.1 Severe hepatic reactions are much less common. These may include jaundice, hepatitis and cholestasis, autoimmune hepatitis, and acute liver failure. It is noteworthy that a number of cases of liver failure resulting in liver transplantation or death have now been reported.2,3 According to the manufacturer of infliximab, at least 35 patients were reported to have had severe hepatic adverse events in the postmarketing period up to 2004.4
Box 3 shows a comparison of our case and several other published cases of postulated infliximab-related AIH, including a case of acute liver failure which led to liver transplantation.2 Neither that patient2 nor our patient received concurrent immunosuppressive therapy during treatment with infliximab. Autoimmune manifestations may possibly be more significant when infliximab is used alone, or in higher doses. This is often the situation with spondyloarthropathy or psoriasis, where additional immunosuppression may not necessarily be part of the protocol. This differs from the treatment of, for example, rheumatoid arthritis, for which patients often also take methotrexate.
One published report describes the development of autoantibodies and liver inflammation following infliximab therapy in two women treated with infliximab for rheumatoid arthritis.2 Liver disease occurred 8 and 17 months, respectively, after infliximab therapy was initiated, and there was de-novo development of ANA in both patients.
Two major types of AIH have been described: type 1 classic AIH, as seen in our patient (related to ANA and/or smooth muscle antibody seropositivity; and type 2 (related to liver/kidney microsomal antibody seropositivity). The histological hallmark of AIH is interface hepatitis, characterised by mononuclear cells, lymphocytes, plasma cells and macrophages infiltrating the portal tract.7
The primary pathogenetic mechanism of AIH is thought to be a loss of tolerance against the patient’s own liver.8 This predominantly periportal hepatitis is thought to be initiated by CD4+ T cells, which recognise self-antigen. Hepatocytes in patients with AIH are postulated to aberrantly express HLA class II molecules on their surface, a process normally involved in the presentation of antigen to CD4+ T cells and other inflammatory cells. Autoimmune disease results from the resultant inflammatory cell activation, with subsequent hepatocyte destruction (cell-mediated autoimmunity).7,8
AIH may also involve the unmodulated production of autoantibodies directed against the hepatocyte membrane (antibody-dependent autoimmunity). This includes elevated immunoglobulin G levels, seen in up to 80% of patients with AIH.7 A genetic predisposition is also thought to be important, with the HLA DR3 and DR4 alleles being associated with the development of type 1 AIH.8
Infliximab is thought to contribute to development of AIH in predisposed patients by triggering development of autoantibodies. These include ANA and anti-dsDNA.9 One possible explanation for this is that TNF-α blockade interferes with the normal cytotoxic T lymphocyte suppression of auto (self)-reactive B cell production. Another is that anti-TNF-α agents interfere with the induced cell death of CD8 T cells, leading to an accentuated lymphocyte presence.9 Studies in patients with rheumatoid arthritis support infliximab-related autoantibody development,9,10 showing elevations in both ANA and anti-dsDNA titres after infliximab therapy.
2 Thin sections of the core liver biopsy sample
- 1. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349: 2014-2022.
- 2. Tobon G, Cañas C, Jaller JJ, et al. Serious liver disease induced by infliximab. Clin Rheumatol 2007; 26: 578-581.
- 3. United States Food and Drug Administration. Safety alerts for drugs, biologics medical devices, and dietary supplements. Remicade (infliximab). Washington, DC: FDA, 2004. http://www.fda.gov/medwatch/SAFETY/2004/safety04.htm#Remicade2 (accessed Sep 2007).
- 4. Everitt DE. Centocor. Important drug warning. December 2004. http://www.fda.gov/medwatch/SAFETY/2004/remicade_DHCP_dec04.pdf (accessed Sep 2007).
- 5. Saleem G, Li SC, MacPherson BR, Cooper SM. Hepatitis with interface inflammation and IgG, IgM and IgA anti double stranded DNA antibodies following infliximab therapy. Arthritis Rheum 2001; 44: 1966-1968.
- 6. Germano V, Picchianti Diamanti A, Baccano G, et al. Autoimmune hepatitis associated with infliximab in a patient with psoriatic arthritis. Ann Rheum Dis 2005; 64: 1519-1520.
- 7. Vergani D, Mieli-Vergani G. Autoimmune hepatitis. Autoimmun Rev 2003; 2: 241-247.
- 8. Diamantis I, Boumpas DT. Autoimmune hepatitis: evolving concepts. Autoimmun Rev 2004; 3: 207-214.
- 9. Eriksson C, Engstrand S, Sundqvist KG, Rantapaa-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF-alpha. Ann Rheum Dis 2005; 64: 403-407.
- 10. Charles PJ, Smeenk RJT, De Jong J, et al. Assessment of antibodies to double stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumour necrosis factor alpha: findings in open label and randomised placebo controlled trials. Arthritis Rheum 2000; 43: 2383-2390.





None identified.