Postpartum haemorrhage (PPH) — excessive bleeding after childbirth — is one of the leading causes of maternal mortality in the developed world.1 The incidence of PPH is increasing.2-4 PPH may arise de novo in any pregnancy, and may recur in a subsequent pregnancy. Previous studies have reported an increased risk of PPH with a previous occurrence, but these studies relied on self-report of previous PPH status,4,5 were hospital-based and not population-based,5,6 involved small numbers of women,7 excluded caesarean deliveries,5-7 and ignored parity.5 The probability that maternal conditions like PPH will occur or recur informs clinical decision making, maternity service provision and guideline development.8
Given Australia’s geography and population distribution, ensuring that women give birth in hospitals that have the facilities to care for them and their babies relies on a system of regionalised care. The ability to identify antenatal risk, allowing transfer of mothers before they give birth, is key to successful regionalised maternity care. Transfer of mothers to appropriate facilities before they give birth has been shown to lower rates of perinatal mortality and morbidity and reduce periods of hospitalisation compared with postnatal–neonatal transfers.9,10 Quantifying the probability of an adverse event will identify mothers who are likely to need increased care and monitoring, thus minimising or avoiding future maternal and perinatal morbidity and mortality.
Data relating to consecutive singleton pregnancies and PPH were obtained from population-based linked birth and hospital-discharge records that were probabilistically linked and de-identified by the New South Wales Department of Health using previously described methods.11,12
PPH was diagnosed during the birth admission by attending obstetricians, clinicians or midwives according to the Australian version of the International classification of diseases guidelines (500 mL or more of blood loss after vaginal delivery or 750 mL or more after a caesarean delivery13,14). Validation of PPH recording against medical records has shown that PPH rates are under-enumerated (sensitivity 58.6%), but with high specificity (99.8%)15 indicating few false-positive reports. We did not include blood transfusions as a marker of severity in our primary definition and analysis of PPH because of a dramatic linear (squared correlation coefficient [R2], 0.92) increase in the year-by-year transfusion rate among women with PPH during the study period (2% in 1994 to 12% in 2002).2 Consequently, the transfusion rate was higher in the first and subsequent pregnancies that occurred later in the study period. However, we undertook a secondary analysis to see if the patterns of occurrence and recurrence were similar when a more stringent definition of PPH (PPH with transfusion) was used while accounting for this background increase in transfusion rates.
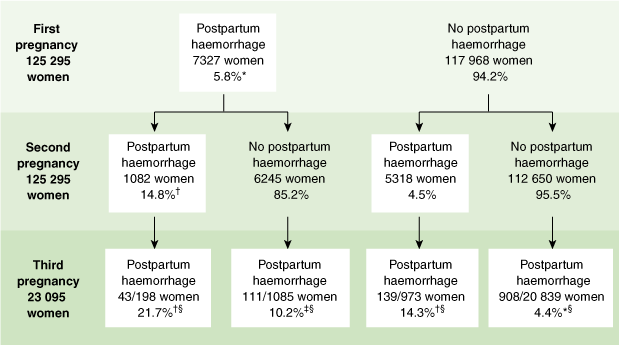
Among the 125 295 women with consecutive pregnancies, 5.8% had a PPH in their first pregnancy, and, of these, 14.8% went on to have a recurrent PPH in their second pregnancy (Box). This represents a risk of recurrent PPH 3.3 times higher than for women with no history of PPH (RR, 3.3; 95% CI, 3.1–3.5). The recurrence risk rose to 21.7% for women who had had two prior PPHs compared with 4.4% for those with no history of PPH (RR, 5.0; 95% CI, 3.8–6.5). Findings were similar when the first PPH occurred in the second pregnancy (4.5%) and then the subsequent pregnancy (14.3%) (Box). In women who had an intervening pregnancy without a PPH (ie, a PPH in a first and third pregnancy only) the risk of PPH recurrence in the third pregnancy was 10.2% (Box).
The strengths of our study include the use of longitudinal population-based data, giving us access to data on rare outcomes16 and the use of validated obstetric items for which accuracy and reliability of reporting have been quantified.15,17 Weaknesses of our study include the inability to assess the severity of PPH and to differentiate between whether the third stage of labour was managed actively (using a prophylactic oxytocic medication before delivery of the placenta, early cord clamping and cutting, controlled umbilical cord traction) or expectantly (allowing the placenta to deliver spontaneously or aided by gravity or nipple stimulation).18 While it is possible that recording of recurrent PPHs is subject to recording bias (having been previously identified as having a PPH may increase the likelihood of further recording), this is unlikely to explain such a large increase in risk.
The recurrence risk we report (RR, 3.3; CI, 3.1–3.5) is very similar to that found in the few previous studies that have been undertaken. The three other studies found that having a history of PPH increased the risk of a recurrence of PPH in a subsequent pregnancy by between 2.2 and 3.3 times compared with women with no history of PPH.5-7 The only other study to investigate recurrence in a third pregnancy found that 25% of women (compared with 22% in our study) with two prior consecutive pregnancies with a PPH went on to have a third PPH.7 It should be noted that all previous studies limited analyses to women with consecutive vaginal deliveries, primarily because quantifying the risk of PPH recurrence was not the primary focus of the study.5-7
We wish to stress that direct comparison of PPH risk by obstetric interventions, such as induction or augmentation of labour or mode of delivery, is problematic. Such interventions are inextricably linked to other risk factors for PPH including maternal medical conditions, placental abnormalities, prolonged labour, genital tract trauma and fetal size. For example, placental abnormalities are associated with PPH risk but are also an indication for caesarean delivery.19 Neither the reasons for interventions, nor the temporal sequence of events can be determined from population health datasets. Therefore, attributing risk to an intervention could be misleading. Consequently, our data on PPH recurrence by mode of delivery should be interpreted with caution. Although the absolute risk of PPH was lower for women having caesarean sections, this may be related to the higher blood loss needed to fulfil the definition of PPH after a caesarean section,13,14 the difficulty estimating blood loss at caesarean section,20 and differential under-reporting in population health datasets by mode of delivery (sensitivity of PPH reporting for vaginal births, 77%; and caesarean sections, 43%).21 Furthermore, stratifying results by mode of delivery results in very small numbers of women with recurrent events, particularly among third pregnancies. We do not believe our study provides sufficient detail on timing and indications for delivery to make recommendations about future mode of delivery. However, importantly, regardless of the mode of delivery there was an increased risk of recurrent PPH.
Longitudinally linked population health data present a unique opportunity to study recurrence risks — using large sample sizes with consistent reporting over time facilitating generalisable results.22 Our cohort could be extended as more data become available, thus increasing the number of women with consecutive births and improving the precision of our recurrence estimates. Furthermore, linkage of birth records to subsequent hospital admissions would enable ascertainment of additional secondary PPHs (occurring between 24 hours and 12 weeks after birth).
As PPH is a major cause of maternal morbidity and mortality,23,24 and the risk of recurrent PPH is high, we suggest that women with a PPH in a prior pregnancy should be delivered in hospitals with onsite blood cross-match facilities. In NSW, only 4% of hospitals providing maternity care do not have cross-match facilities (all are small rural hospitals) and, in making this recommendation, we calculate that only 178 (2.1/1000) of the 85 000 women giving birth in NSW would be affected by a policy recommending delivery in a hospital with an onsite blood-bank for women with a prior PPH.
Reporting the risks of recurrent PPH enables informed risk-counselling of pregnant women about the most appropriate place to give birth and the need for rigorous application of active management of the third stage of labour.18 If one in seven women will have a second PPH and one in five women who have had a second will have a third PPH, consideration should be given to subsequent delivery in a hospital that has onsite cross-match facilities.
Received 13 February 2007, accepted 16 July 2007
- Jane B Ford1
- Christine L Roberts1
- Jane C Bell1
- Charles S Algert2
- Jonathan M Morris3
- 1 Kolling Institute of Medical Research, Northern Clinical School, University of Sydney, Sydney, NSW.
- 2 The George Institute for International Health, Sydney, NSW.
- 3 Department of Obstetrics and Gynaecology, Northern Clinical School, University of Sydney, Sydney, NSW.
We acknowledge the efforts of the hospital staff who collect the data, and NSW Health Department staff for their role in the design and maintenance of the HOIST data warehouse system, and data linkage. Jane Ford is supported by the Health Research and Outcomes Network, a National Health and Medical Research Council (NHMRC) Capacity Building Grant in Population Health Research. Christine Roberts is supported by an NHMRC Senior Research Fellowship.
None identified.
- 1. World Health Organization Department of Reproductive Health and Research. Maternal mortality in 2000: estimates developed by WHO, UNICEF, and UNFPA. Geneva: WHO, 2004.
- 2. Cameron CA, Roberts CL, Olive EC, et al. Trends in postpartum haemorrhage. Aust N Z J Public Health 2006; 30: 151-156.
- 3. Ford JB, Roberts CL, Simpson JM, et al. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet 2007; 98: 237-243.
- 4. Joseph KS, Rouleau J, Kramer M, et al, for the Maternal Health Study Group of the Canadian Perinatal Surveillance System. Investigation of an increase in postpartum haemorrhage in Canada. BJOG 2007; 114: 751-759.
- 5. Magann EF, Evans S, Hutchinson M, et al. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J 2005; 98: 419-422.
- 6. Combs CA, Murphy EL, Laros RK. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol 1991; 77: 69-76.
- 7. Hall MH, Halliwell R, Carr-Hill R. Concomitant and repeated happenings of complications of the third stage of labour. Br J Obstet Gynaecol 1985; 92: 732-738.
- 8. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA 1999; 281: 1214-1219.
- 9. Sanderson M, Sappenfield WM, Jespersen KM, et al. Association between level of delivery hospital and neonatal outcomes among South Carolina Medicaid recipients. Am J Obstet Gynecol 2000; 183: 1504-1511.
- 10. Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA 1996; 276: 1054-1059.
- 11. Ford JB, Roberts CL, Taylor LK. Characteristics of unmatched maternal and baby records in linked birth records and hospital discharge data. Paediatr Perinat Epidemiol 2006; 20: 329-337.
- 12. Taylor LK, Simpson JM, Roberts CL, et al. Risk of complications in a second pregnancy following caesarean section in the first pregnancy: a population-based study. Med J Aust 2005; 183: 515-519. <MJA full text>
- 13. National Centre for Classification in Health. Australian coding standards for ICD-10-AM and ACHI. Sydney: NCCH, 2006.
- 14. National Coding Centre. The Australian version of the international classification of diseases, 9th revision, clinical modification (ICD-9-CM). Sydney: University of Sydney, 1996.
- 15. Taylor L, Travis S, Pym M, et al. How useful are hospital morbidity data for monitoring conditions occurring in the perinatal period? Aust N Z J Obstet Gynaecol 2005; 45: 36-41.
- 16. Bright R, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies: strengths and limitations. J Clin Epidemiol 1989; 42: 937-945.
- 17. NSW Health Department. Validation study: NSW Midwives Data Collection 1998. New South Wales Mothers and Babies 1998. NSW Public Health Bull 2000; 9 (S-2): 97-99. (State Publication no. [EPI] 000029.) http://www.health.nsw.gov.au/public-health/mdc/mdcrep98.html (accessed Aug 2007).
- 18. Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database Syst Rev 2000; (3): CD000007.
- 19. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. First-birth cesarean and placental abruption or previa at second birth. Obstet Gynecol 2001; 97: 765-769.
- 20. Burrows LJ, Meyn LA, Weber AM. Maternal morbidity associated with vaginal versus cesarean delivery. Obstet Gynecol 2004; 103: 907-912.
- 21. Roberts CL, Lain S, Hadfield R. Quality of population health data reporting by mode of delivery. Birth 2007; 34: 274-275.
- 22. Ananth CV, Getahun D, Peltier MR, et al. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006; 195: 643-650.
- 23. Slaytor EK, Sullivan EA, King JF. Maternal deaths in Australia, 1997–1999. Sydney: Australian Institute of Health and Welfare National Perinatal Statistics Unit, 2004.
- 24. Hebert PR, Reed G, Entman SS, et al. Serious maternal morbidity after childbirth: prolonged hospital stays and readmissions. Obstet Gynecol 1999; 94: 942-947.





Abstract
Objective: To determine the risk of occurrence and recurrence of postpartum haemorrhage (excessive bleeding after childbirth) among women having at least two consecutive pregnancies.
Design and setting: Population-based study using longitudinally linked hospital discharge and birth records from New South Wales for the period 1 January 1994 to 31 December 2002.
Participants: All 125 295 women having at least a first and second pregnancy resulting in a singleton birth at > 400g or ≥ 20 weeks’ gestation in the study period.
Main outcome measures: Risk of occurrence of postpartum haemorrhage (PPH) in any pregnancy, and of recurrence of PPH in subsequent (second and third) pregnancies.
Results: 5.8% of women (7327/125 295) had a PPH in their first pregnancy, and 4.5% (5318/117 968) had a first PPH in their second pregnancy. Among the 23 095 women who had three pregnancies in the study period, 4.4% (908/20 839) had a first PPH in their third pregnancy. The risk of recurrence in a second consecutive pregnancy was 14.8% (1082/7327), and in a third consecutive pregnancy (after two previous PPHs) was 21.7% (43/198); even with an intervening pregnancy with no PPH (ie, PPH in the first and third pregnancies only), the risk for the third pregnancy was 10.2% (111/1085).
Conclusions: These consistently elevated risks of recurrence highlight the need for women with a history of PPH to have active management of the third stage of labour and to give birth in a hospital that has onsite blood cross-match facilities.