Lung transplantation (LTx) in adolescents is limited in Australia and worldwide by both low numbers of adolescent and small organ donors, and a relatively low rate of referral for LTx in this age group. The first reported series of LTx in children and adolescents came in the late 1980s from the United Kingdom and Europe,1,2 and the largest single-centre series from the St Louis Children’s Hospital in the United States, where 207 lung transplants were performed in 190 children between 1990 and 2002.3 The 2006 International Society for Heart and Lung Transplantation (ISHLT) Registry reported a plateauing in the number of transplants in the paediatric age range in the past 5 years, with about 40 per annum in 11–17-year-olds, giving a worldwide total of 382 since 1995.4,5
We performed a prospective cohort study of all adolescent patients who had heart–lung transplantation (HLTx) or LTx at St Vincent’s Hospital. Selection criteria for LTx were according to international guidelines.6,7
Characteristics of the 37 recipients before transplantation are shown in Box 1. Demographic characteristics relevant to surgery for the recipients are shown in Box 2.
Serological cytomegalovirus (CMV) and Epstein–Barr virus (EBV) status were established in all donors and recipients. Patients seronegative for CMV who were transplanted with a graft from a CMV-seropositive donor (CMV “mismatched” recipients) received antiviral prophylaxis with intravenous ganciclovir (5 mg/kg three times per week) for 10 weeks. CMV pneumonia was defined by the presence of typical inclusion bodies with cytopathic effect on transbronchial lung biopsies, and treated with intravenous ganciclovir (5 mg/kg, twice a day for 14–21 days ± 10 mg/kg/day three times a week for the next 14 days).8 Patients underwent routine surveillance bronchoscopy with transbronchial lung biopsy at 3, 6 and 9–12 weeks after transplantation, with additional procedures for new-onset symptoms or as a follow-up for acute rejection or CMV pneumonia, as described previously.9 Transbronchial lung biopsies were performed as described previously,10 and biopsy findings were classified according to standard ISHLT nomenclature.11 Pulmonary function tests were performed according to the guidelines of the American Thoracic Society.12 BOS was defined according to the updated recommendations of the ISHLT.13
Data were entered prospectively into the St Vincent’s Lung Transplantation database and were analysed using SPSS version 14.0 (SPSS Inc, Chicago, Ill, USA). Data were expressed as mean ± SD and range if normally distributed, and median and interquartile range (IQR) if not. For the comparison between means, the independent t test was used for parametric data and the Mann–Whitney U test for non-parametric data. The χ2 test was performed for categorical variables. Kaplan–Meier survival curves were compared with the Mantel–Cox log-rank test. To examine potential variables that might alter the hazard of post-transplantation death and the risk of BOS, we chose variables that have been reported previously as potential risk factors.4,5 Univariate Cox proportional hazards models were constructed using each variable, and a final candidate multivariate model was constructed using the three variables with significant univariate risks for BOS. There were insufficient numbers of recipients to meet criteria for a more comprehensive Cox proportional hazards multivariate analysis. For all tests, P < 0.05 was considered significant.
The mean waiting time to transplant was 273 days (range, 5–964 days; median, 163 days) and the mean donor age was 28 years (Box 2). Fewer than half the donors were aged less than 20 years. Fifty-one per cent of recipients were seronegative for CMV, compared with 27% of donors, and 37% of recipients were EBV-naïve, only one of whom developed post-transplant lymphoproliferative disease. In total, 216 transbronchial lung biopsies were performed in 36 patients (mean, 6.0 ± 3.7 biopsies per patient; range, 1–14). Overall, 18 of 35 evaluable patients13 (51.4%) developed BOS.
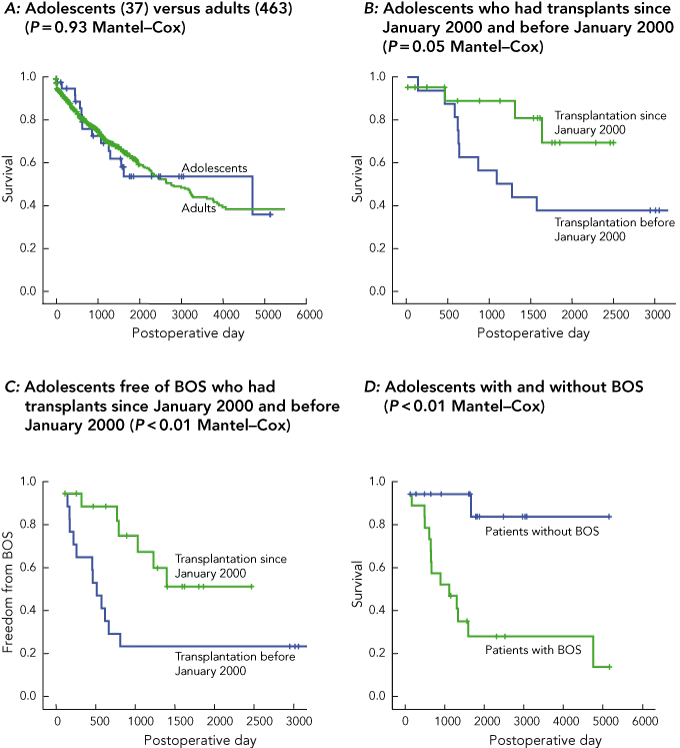
For all 37 adolescents transplanted, the perioperative (30-day), 1-year, 5-year and 10-year survival rates were 97% ± 3%, 95% ± 4%, 55% ± 9% and 55% ± 9%, respectively, compared with 96% ± 1%, 88% ± 2%, 64% ± 2% and 45% ± 3% for 463 contemporaneous adult recipients (P = 0.93; Box 3A). The sole perioperative death in the adolescent group was caused by cerebral thrombosis, which occurred after the insertion of a right ventricular support device required for acute right heart failure associated with primary graft dysfunction. We would now manage this situation with ECMO.
Overall, 15 of the 37 adolescent lung transplant recipients have died in this 16-year period; 12 of the 15 deaths were caused by respiratory failure associated with BOS with or without bronchopulmonary sepsis, at a mean postoperative day of 1078. One patient with refractory post-transplant lymphoproliferative disease died from sepsis after chemotherapy. The 5-year survival for the 16 patients transplanted before January 2000 was 38%, compared with 74% for the 21 patients transplanted subsequently (Box 3B). BOS-free survival was significantly higher in the 21 patients who had transplants since January 2000 (P < 0.01; Mantel–Cox; Box 3C). Freedom from BOS was associated with a reduced mortality risk (hazard ratio, 0.07; 95% CI, 0.01–0.52; P < 0.01; Box 3D). On univariate analysis, none of the following variables was associated with a significant excess risk of mortality after LTx: recipient age (P = 0.24); donor age (P = 0.84); CMV mismatch (P = 0.64); sex of the donor (P = 0.17); sex of the recipient (P = 0.74); donor/recipient sex mismatch (P = 0.84); ischaemic time (P = 0.96); pretransplant diagnosis (P = 0.72); or weight (P = 0.54). Univariate risks for BOS were: increasing recipient age within the range of 12–19 years (hazard ratio, 1.44; 95% CI, 1.10–1.89; P = 0.01), male sex of donor (hazard ratio, 2.69; 95% CI, 1.03–7.00; P = 0.04); and LTx before January 2000 (hazard ratio, 3.45; 95% CI, 1.29–9.27; P = 0.01).
Several groups have reported excellent survival rates after lung transplantation in paediatric patients compared with adults.14-17 The results of a Spanish study are similar to our own, with an 8-year survival of 62% for 23 paediatric recipients aged less than 16 years versus 41% for 142 adult patients defined as aged over 16 years.14 The largest reported series of 207 lung transplants in 190 children transplanted from 1990–2002 at St Louis Children’s Hospital showed a 1-year survival of 77%, with 55% alive at 5 years.3 A recent report from the UK showed a 100% 1-year survival for 23 paediatric patients who had LTx in 2002–2005 (median age, 14 years).18 Our overall results demonstrating a 95% 1-year and 55% 5-year survival compare favourably with the international results.
Another UK study showed a clear survival benefit for children with CF who underwent LTx,19 and this analysis was based on similar listing criteria and donor organ allocation processes as those in Australia. A recent statistical analysis that used two large US registry populations to predict survival advantage for patients with CF undergoing LTx concluded that transplantation “. . . never improves survivorship for paediatric patients . . . our results may suggest a rigid cut-off age of 18 for LTx”. However, this model did not consider oxygen dependency, blood gas data, exercise capacity or desaturation on a 6-minute or 12-minute walk test.20 Centres in Australia, like those in the UK, prioritise sicker patients for LTx, in contradistinction to the “time waited” approach traditionally used for organ allocation in the US. Indeed, there could be little doubt that our nine patients who were dependent on non-invasive ventilation (five), invasive ventilation (two) or ECMO (two) derived a real survival benefit from LTx, as the latter four patients, in particular, were within days of death. Furthermore, our improved 5-year survival rate of 74% in patients transplanted since January 2000 (Box 3B) is significantly above that of the ISHLT Registry benchmark of 47%.4
Lack of suitably sized donors is the dominant factor determining the availability of transplantation for adolescents. Lobar transplantation from living related donors is well established, but limited to groups with expertise in this area.21 There is no Australian program for living related donors for LTx at this time. The Alfred Hospital in Melbourne has developed protocols for lung retrieval from “donors after cardiac death”, in addition to the traditional donation after brain death, and this will further increase the donor pool in Australia.22
Factors contributing to our improved survival rates documented since 2000 include evolving strategies in many areas associated with long-term survival detailed below. Chronic renal disease largely related to calcineurin-inhibitor toxicity is a significant problem in paediatric (and adult) LTx, and has been shown to be most dramatic in the adolescent age range.23 Strategies to prevent chronic renal impairment and end stage renal disease by C2 monitoring (2-hour post-dose monitoring of cyclosporin levels) have been a particular focus of our unit.24,25 C2 monitoring has replaced the traditional trough-level monitoring of cyclosporin (C0 monitoring) in our unit since 2000, with the developing body of evidence in solid organ transplantation showing superior outcomes. Furthermore, we have shown that C2 monitoring can reduce the incidence of early acute cellular rejection, which is one of the biggest risk factors for BOS, the leading cause of death in long-term survivors of LTx.26 Missed rejection could also be relevant, despite our rigorous transbronchial lung biopsy surveillance program. Even minimal rejection has been shown to increase the risk of BOS.27 Thus, we have not modified our transbronchial lung biopsy surveillance program in this younger age group, and have not experienced increased difficulties or complications of the procedure compared with when it is performed in adults. The recent series from the UK, with 100% 1-year survival of its paediatric lung transplant recipients, used a bronchoscopy surveillance protocol similar to our own, but extending out further to 12 months after LTx, and draws the same conclusions about its clinical utility and safety in this age group.18 Our experience since January 2000 shows more patients are remaining BOS-free (Box 3C), and computing their likely survival based on our experience of BOS-free survival to date (Box 3D) provides evidence for guarded optimism.
Early series of LTx in younger age groups report an increased incidence of CMV-mismatched and EBV-mismatched grafts, largely because of the low rate of prior CMV and EBV exposure in recipients before transplantation.28,29 The advent of antiviral agents specifically targeted against these viruses has reduced the impact of these infections as serious problems after LTx, particularly with prophylactic strategies using ganciclovir or valganciclovir against CMV. Also, avoidance of induction therapy (since 1995) and long-term treatment with aciclovir or valaciclovir (introduced in 1998) significantly reduces the incidence of post-transplant lymphoproliferative disease in EBV-naïve recipients.30
There are no LTx reports that differentiate outcomes between paediatric and adolescent age groups, but reports in other solid organ transplant recipients identify decreased adherence to therapy among adolescents, resulting in an increased incidence of late acute rejection and chronic rejection, with reduced survival in adolescents compared with both paediatric and adult recipients.31 Our results mirror these findings, as increasing age (within the age range 12–19 years) was associated with an increased risk of BOS on both univariate and multivariate analysis.
1 Characteristics of 37 adolescents before heart–lung or lung transplantation during 1991–2006 at St Vincent’s Hospital, Sydney
- Judith M Morton1
- Monique A Malouf2
- Marshall L Plit2
- Phillip M Spratt2
- Allan R Glanville2
- 1 Respiratory and Sleep Medicine, Monash Medical Centre, Melbourne, VIC.
- 2 Lung Transplant Unit, Department of Thoracic Medicine, St Vincent’s Hospital, Sydney, NSW.
We gratefully acknowledge all members of the Lung Transplant Unit who have assisted in the care of these patients and, in particular, our patients described herein.
None identified.
- 1. Smyth RL, Higenbottam TW, Scott JP, McGoldrick JP, et al. Early experience of heart–lung transplantation. Arch Dis Child 1989; 64: 1225-1229; discussion 1229-1230.
- 2. Vouhe PR, Le Bidois J, Dartevelle P, et al. Heart- and heart–lung transplantation in children. Eur J Cardiothorac Surg 1989; 3: 191-195.
- 3. Huddleston CB, Bloch JB, Sweet SC, et al. Lung transplantation in children. Ann Surg 2002; 236: 270-276.
- 4. Waltz DA, Boucek MM, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric lung and heart–lung transplantation report — 2006. J Heart Lung Transplant 2006; 25: 904-911.
- 5. Trulock EP, Edwards LB, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart–lung transplantation report — 2006. J Heart Lung Transplant 2006; 25: 880-892.
- 6. Glanville AR, Estenne M. Indications, patient selection and timing of referral for lung transplantation. Eur Respir J 2003; 22: 845-852.
- 7. Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update — a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006; 25: 745-755.
- 8. Tamm M, Aboyoun CL, Chhajed PN, et al. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2004; 170: 1120-1123.
- 9. Aboyoun CL, Tamm M, Chhajed PN, et al. Diagnostic value of follow-up transbronchial lung biopsy after lung rejection. Am J Respir Crit Care Med 2001; 164: 460-463.
- 10. Hopkins PM, Aboyoun CL, Chhajed PN, et al. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant 2002; 21: 1062-1067.
- 11. Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996; 15(1 Pt 1): 1-15.
- 12. Standardization of spirometry — 1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987; 136: 1285-1298.
- 13. Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002; 21: 297-310.
- 14. Alvarez A, Algar FJ, Santos F, et al. Pediatric lung transplantation. Transplant Proc 2005; 37: 1519-1522.
- 15. Conte JV, Robbins RC, Reichenspurner H, et al. Pediatric heart–lung transplantation: intermediate-term results. J Heart Lung Transplant 1996; 15: 692-699.
- 16. Sweet SC. Pediatric lung transplantation: update 2003. Pediatr Clin North Am 2003; 50: 1393-1417, ix.
- 17. Mendeloff EN. Pediatric lung transplantation. Chest Surg Clin N Am 2003; 13: 485-504.
- 18. Benden C, Harpur-Sinclair O, Ranasinghe AS, et al. Surveillance bronchoscopy in children during the first year after lung transplantation: is it worth it? Thorax 2007; 62: 57-61.
- 19. Aurora P, Whitehead B, Wade A, et al. Lung transplantation and life extension in children with cystic fibrosis. Lancet 1999; 354: 1591-1593.
- 20. Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med 2005; 171: 1053-1059.
- 21. Starnes VA, Woo MS, MacLaughlin EF, et al. Comparison of outcomes between living donor and cadaveric lung transplantation in children. Ann Thorac Surg 1999; 68: 2279-2283; discussion 2283-2284.
- 22. Oto T, Levvey B, McEgan R, et al. A practical approach to clinical lung transplantation from a Maastricht Category III donor with cardiac death. J Heart Lung Transplant 2007; 26: 196-199.
- 23. Hmiel SP, Beck AM, de la Morena MT, Sweet S. Progressive chronic kidney disease after pediatric lung transplantation. Am J Transplant 2005; 5: 1739-1747.
- 24. Morton JM, Aboyoun CL, Malouf MA, et al. Enhanced clinical utility of de novo cyclosporine C2 monitoring after lung transplantation. J Heart Lung Transplant 2004; 23: 1035-1039.
- 25. Glanville AR, Morton JM, Aboyoun CL, et al. Cyclosporine C2 monitoring improves renal dysfunction after lung transplantation. J Heart Lung Transplant 2004; 23: 1170-1174.
- 26. Glanville AR, Aboyoun CL, Morton JM, et al. Cyclosporine C2 target levels and acute cellular rejection after lung transplantation. J Heart Lung Transplant 2006; 25: 928-934.
- 27. Hopkins PM, Aboyoun CL, Chhajed PN, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 2004; 170: 1022-1026.
- 28. Boyle GJ, Michaels MG, Webber SA, et al. Posttransplantation lymphoproliferative disorders in pediatric thoracic organ recipients. J Pediatr 1997; 131: 309-313.
- 29. Armitage JM, Kurland G, Michaels M, et al. Critical issues in pediatric lung transplantation. J Thorac Cardiovasc Surg 1995; 109: 60-64; discussion 64-65.
- 30. Malouf MA, Chhajed PN, Hopkins P, et al. Anti-viral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J Heart Lung Transplant 2002; 21: 547-554.
- 31. Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant 2005; 9: 381-390.





Abstract
Objective: To describe the results of lung transplantation (LTx) in adolescents at a hospital for adults.
Design and setting: Prospective cohort study set in an LTx unit at an adult tertiary referral hospital from 1991 to 2006.
Patients: 37 consecutive adolescent lung transplant recipients including 13 males and 24 females (mean age, 16.7 ± 2.0 [SD] years; range 12–19 years) who received heart–lung (six patients) or bilateral LTx (31 patients) for cystic fibrosis (29), congenital heart disease (four), acute respiratory failure (two), or another disorder (two). Two patients were transplanted after invasive ventilation, five after non-invasive ventilation and two after extracorporeal membrane oxygenation.
Main outcome measures: Overall survival compared with an adult cohort; survival free of bronchiolitis obliterans syndrome (BOS); overall and BOS-free survival in those transplanted before and after January 2000.
Results: Mean waiting time was 273 days (range, 5–964 days; median, 163 days), mean donor age was 28 years (range, 9–53 years). Median inpatient stay was 11 days (range, 7–94 days). Mean follow-up was 1540 ± 1357 days (range, 35–5163 days). The 5-year survival rate for the 16 patients transplanted before January 2000 was 38%, versus 74% for the 21 transplanted since January 2000 (P = 0.05; Mantel–Cox). Overall, 18 of 35 evaluable patients developed BOS. Only BOS was associated with an increased mortality risk (P < 0.01).
Conclusion: LTx may be performed successfully in adolescents at a hospital for adults.