Bairnsdale or Buruli ulcer (BU) is an ulcerative skin and soft tissue infection caused by Mycobacterium ulcerans, and predominantly occurs in sub-Saharan Africa.1,2 Although small foci exist in East Gippsland (Victoria) and Queensland, most Australian cases have occurred in coastal Victorian towns.3,4 Risk factors for BU have been investigated, but the mode of transmission remains unknown.5-8 Late diagnosis of BU contributes to significant morbidity and treatment costs.2,9
In Victoria, the incidence of BU is increasing, from four cases in 1999, to 61 notified cases in 2006. Half the cases in 2006 were linked to the Bellarine Peninsula, where BU was first described in 1998.10,11 Within the Bellarine Peninsula, most cases have been clustered in the towns of St Leonards (since 1998), Point Lonsdale (since 2002) and Barwon Heads/Ocean Grove (since 2005).
Patients diagnosed with BU between January 1998 and August 2006 were identified retrospectively through medical records of physicians in the region (including 40 patients whose diagnosis and management have been described previously11) or the Victorian Department of Human Services (DHS) notifiable diseases database (BU became notifiable in 2004). BU was diagnosed by a positive M. ulcerans culture and/or polymerase chain reaction from a clinical specimen.12 Only patients epidemiologically linked to the Bellarine Peninsula were included. Patients were excluded if they could not be contacted (3), had dementia (1) or had died (1).
Statistical analysis was completed using Minitab, version 14 (Minitab Inc, State College, Pa, USA). Comparison of the time from first onset of symptoms to seeking medical attention, and from first medical appointment to diagnosis was completed using the Kruskal–Wallis or Mann–Whitney test. The Edwards test for seasonal trends13 was used to determine the periodicity of monthly totals for incident cases identified as time of onset of BU symptoms. An estimated incidence was calculated using patients who resided permanently on the Bellarine Peninsula and the 2001 Australian census data.
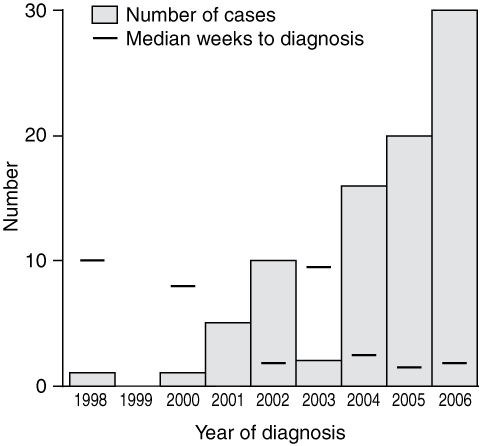
Of 110 eligible patients, 85 participated (77% participation rate). The median time between diagnosis of BU and completion of the questionnaire was 6 months (interquartile range [IQR], 2.0–14.3 months). There were 45 male and 40 female participants (median age: men, 63 years [IQR, 36–72 years], women, 81 years [IQR, 51–85 years]; P < 0.01), and most cases occurred in 2006 (n = 30) (Box 1). Sixty-one patients resided in the endemic regions. For patients resident in the endemic area, the estimated annual incidence of BU for the period March 1998 to August 2006 was 3.1 per 10 000 people.
Twenty-eight patients had lesions on the arm, and 51 on the leg (Box 2). Forty-one patients described a papule, 19 a small ulcer, and seven an eschar as the first symptom. Ten patients recalled an injury before BU symptoms, and 17 recalled an insect bite; however, most patients were unable to report how much time had elapsed from this until the onset of BU symptoms.
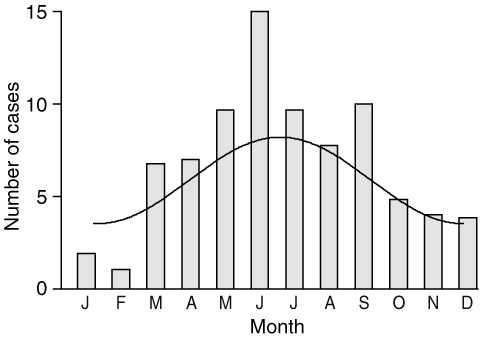
The monthly variation in the date of first symptoms was best described by a simple harmonic curve (P < 0.01) (Box 3), with the trough in early January and the peak in early July. Based on incubation periods of three patients who reported specific timing of exposure relating to stays on the Bellarine Peninsula limited to a week, we estimate that the latent period was 1–4 months.
Nineteen patients cited viewing media reports of BU as a significant factor in seeking medical attention (Box 2). The median time between patients first noticing symptoms and seeking medical attention was 3.5 weeks (IQR, 1.0–8.0 weeks) and by location ranged from 2 to 6 weeks (Box 4). This time was shorter for people living in endemic regions (3.0 weeks; IQR, 1.0–5.0 weeks) than in non-endemic areas (5.3 weeks; IQR, 2.0–9.5 weeks) (P = 0.05).
The median time between the first medical consultation and BU diagnosis was 2.0 weeks (IQR, 0.0–6.0 weeks). For residents of Point Lonsdale and St Leonards, the delay from presentation to diagnosis was less than for Barwon Heads and Greater Victoria (P = 0.01) (Box 4). Patients who resided in an endemic area had a shorter median time to diagnosis (1.0 week; IQR, 0.0–3.9 weeks) than those who resided in non-endemic areas (5.0 weeks; IQR, 1.3–8.0 weeks) (P = 0.001). There was a decrease in time elapsed for cases diagnosed later in the outbreak (P = 0.23) (Box 1).
Thirty patients were diagnosed with BU at the first consultation with their general practitioner. Alternative initial diagnoses included non-specific infection and spider bite; for 22 patients, no diagnosis was made at the first visit (Box 2). Thirty-seven patients reported a test was ordered for BU, and 40 patients had medication prescribed. More than three-quarters were prescribed non-antimycobacterial oral antibiotics; topical treatments were prescribed for the others.
In our series of patients with BU on the Bellarine Peninsula, 30% lived outside the endemic area. Previous research has highlighted the importance of increased awareness of BU symptoms in affected communities in reducing the delay in presentation to health care providers and reducing illness severity and treatment costs.9,14 In this study, a delay of 2–6 weeks occurred from first symptoms to seeking medical treatment. This is similar to the elapsed time in southern Benin, where a national program aimed at improving the detection of BU reduced the time from 9 months to 1 month before patients sought medical attention.2 Although BU still has a low profile in Australia, most residents on the Bellarine Peninsula have been made aware of it through DHS alerts between 2002 and 2006 (two residential alerts to Point Lonsdale and one to St Leonards, and six general alerts to GPs), and extensive media coverage in the regions. This is likely to have contributed to the comparative timeliness of presentation.
Although 60 patients first noticed a papule or small ulcer, most of these patients delayed seeking care until the lesion had deteriorated. It is possible that the painless nature, slow progression and misinterpretation of symptoms were the main reasons for not seeking medical attention sooner. Most patients were not diagnosed at the first consultation, and diagnosis took longer in those who resided outside the Bellarine Peninsula. It is likely that this delay resulted from low awareness in patients and doctors, especially outside the endemic regions. Time trends also suggest that BU was recognised more rapidly as the epidemic unfolded on the Bellarine Peninsula. These findings support the need to raise awareness in newly endemic areas, and to concentrate efforts on alerting those outside endemic areas.15
There has been a steady increase in the annual number of cases over the past 5 years, with new cases linked to Barwon Heads; this could indicate a continuing spread of the bacteria across the Bellarine Peninsula through a change in vector or environmental conditions. The month in which patients first noticed symptoms peaked in mid winter, although symptoms occurred throughout the year. Thus, emphasis on awareness campaigns during winter may be warranted. Of note, the first seasonal rainfall peak on the Bellarine Peninsula, from April to May, closely precedes the peak incidence of BU.16 Rainfall events trigger increased mosquito activity, and there is increasing evidence that mosquito exposure may be linked to BU.17,18
Our study was limited by the accuracy of patient recall. However, validation of the time elapsed from first symptoms to the doctor’s diagnosis was aided by DHS notification dates and contemporaneous notes made by physicians. There is uncertainty regarding our estimated BU incidence, as the regional population varies greatly during the year, peaking during summer, and we cannot exclude misdiagnoses,19-21 or underreporting of cases to the DHS. We also acknowledge the uncertainty of the incubation period derived based on three patients; incubation periods of 3 and 7 months have been reported elsewhere.22
2 Clinical characteristics of presentation and initial diagnosis for Bairnsdale ulcer for 85 patients
Received 5 April 2007, accepted 9 August 2007
- Tricia Y J Quek1
- Margaret J Henry1
- Julie A Pasco1
- Daniel P O’Brien1,2
- Paul D R Johnson3
- Andrew Hughes1
- Allen C Cheng1
- Jane Redden-Hoare1
- Eugene Athan1
- 1 Barwon Health, University of Melbourne, Geelong, VIC.
- 2 Public Health Department, Médecins Sans Frontières Holland, Amsterdam, The Netherlands.
- 3 Austin Health, Melbourne, VIC.
None identified.
- 1. Amofah G, Bonsu F, Tetteh C, et al. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis 2002; 8: 167-170.
- 2. Debacker M, Aguiar J, Steunou C, et al. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg Infect Dis 2004; 10: 1391-1398.
- 3. MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol 1948; 60: 93-122.
- 4. Johnson PD, Veitch MG, Leslie DE, et al. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust 1996; 164: 76-78.
- 5. Aiga H, Amano T, Cairncross S, et al. Assessing water-related risk factors for Buruli ulcer: a case–control study in Ghana. Am J Trop Med Hyg 2004; 71: 387-392.
- 6. Debacker M, Portaels F, Aguiar J, et al. Risk factors for Buruli ulcer, Benin. Emerg Infect Dis 2006; 12: 1325-1331.
- 7. Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case–control study in Ghana. Clin Infect Dis 2005; 40: 1445-1453.
- 8. Marston BJ, Diallo MO, Horsburgh CR Jr, et al. Emergence of Buruli ulcer disease in the Daloa region of Cote d’Ivoire. Am J Trop Med Hyg 1995; 52: 219-224.
- 9. Drummond C, Butler JR. Mycobacterium ulcerans treatment costs, Australia. Emerg Infect Dis 2004; 10: 1038-1043.
- 10. Victorian Department of Human Services. Notifications of infectious diseases: Victorian summary report 1 January – 30 January 2007. Melbourne: Communicable Disease Control, Public Health Branch, 2007.
- 11. O’Brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australian case series. Med J Aust 2007; 186: 58-61. <MJA full text>
- 12. Ross BC, Marino L, Oppedisano F, et al. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 1997; 35: 1696-1700.
- 13. Edwards JH. The recognition and estimation of cyclic trends. Ann Hum Genet 1961; 25: 83-87.
- 14. Johnson PD, Stinear T, Small PL, et al. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med 2005; 2: e108.
- 15. Johnson PDR, Hayman JA, Quek TY, et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust 2007; 186: 64-68. <MJA full text>
- 16. Geelong Weather Services. Summary of Geelong weather. http://users.pipeline.com.au/~gws/archives.htm (accessed Feb 2007).
- 17. Johnson PDR, Azuolas J, Lavender CJ, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis 2007; Nov. [Epub ahead of print] http://www.cdc.gov/eid/content/13/11/pdfs/06-1369.pdf (accessed Oct 2007).
- 18. Quek TYJ, Athan E, Henry MJ, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis 2007; Nov. [Epub ahead of print] http://www.cdc.gov/eid/content/13/11/pdfs/06-1206.pdf (accessed Oct 2007).
- 19. Amofah GK, Sagoe-Moses C, Adjei-Acquah C, Frimpong EH. Epidemiology of Buruli ulcer in Amansie West district, Ghana. Trans R Soc Trop Med Hyg 1993; 87: 644-645.
- 20. van der Werf TS, van der Graaf WT, Groothuis DG, Knell AJ. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans R Soc Trop Med Hyg 1989; 83: 410-413.
- 21. Siegmund V, Adjei O, Nitschke J, Thompson WA. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis 2007; 45: 68-75.
- 22. Lavender CJ, Senanayake SN, Fyfe JAM, et al. First case of Mycobacterium ulcerans disease (Bairnsdale or Buruli ulcer) acquired in New South Wales. Med J Aust 2007; 186: 62-63. <MJA full text>





Abstract
Objective: To document the epidemiology, clinical characteristics and diagnosis of an outbreak of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer [BU]) during the period 1998–2006, and compare delays in diagnosis between residents of endemic and non-endemic regions.
Design and setting: Retrospective case study of patients identified through infectious disease physicians on the Bellarine Peninsula and the Victorian Department of Human Services notifiable diseases database.
Main outcome measures: Description of events leading to diagnosis of BU.
Results: Eighty-five BU patients recalled their experience. Fifty-three patients were older than 60 years, and 61 permanently resided on the Bellarine Peninsula. The onset of symptoms occurred most frequently in mid winter. Twenty-eight patients had lesions on the arm and 51 on the leg. The median time between onset of symptoms and first medical contact was shorter for those living in the endemic area (3.0 weeks; interquartile range [IQR], 1.0–5.0 weeks) compared with non-endemic areas (5.3 weeks; IQR, 2.0–9.5 weeks) (P = 0.05). Patients who resided in the endemic area had a shorter median time from their first medical appointment to diagnosis (1.0 week; IQR, 0.0–3.9 weeks) than those who resided in non-endemic areas (5.0 weeks; IQR, 1.3–8.0 weeks) (P = 0.001).
Conclusion: Delay in presentation and time to diagnosis of BU are longer in non-endemic than endemic areas. Measures should be taken to raise awareness of the disease in non-endemic areas.