Enterococci are normal flora of the gastrointestinal tract. Over the past 10–15 years, there has been a rapid increase in the prevalence of vancomycin-resistant enterococci (VRE).1,2 The two most common species, Enterococcus faecalis and E. faecium, can harbour vanA and vanB genes, which encode resistance to vancomycin and have been implicated in the development of persistent, transmissible nosocomial infections that may be associated with poor outcomes.3 The increasing prevalence of VRE has been associated with widespread use of broad-spectrum antibiotics and with cross-infection.4 Because of the ability of VRE to transfer their antibiotic resistance factors to other microorganisms, and the threat of clinical infections with VRE in susceptible patient groups, prevention and control measures are critical.
Probiotics are living microbial food ingredients designed to have a beneficial effect on human health. As almost all strains of lactobacilli are resistant to vancomycin5,6 and probiotics have been used with some success in preventing colonisation by enteric pathogens during treatment for Clostridium difficile,7 we attempted to determine whether probiotics might reduce bowel colonisation by VRE.
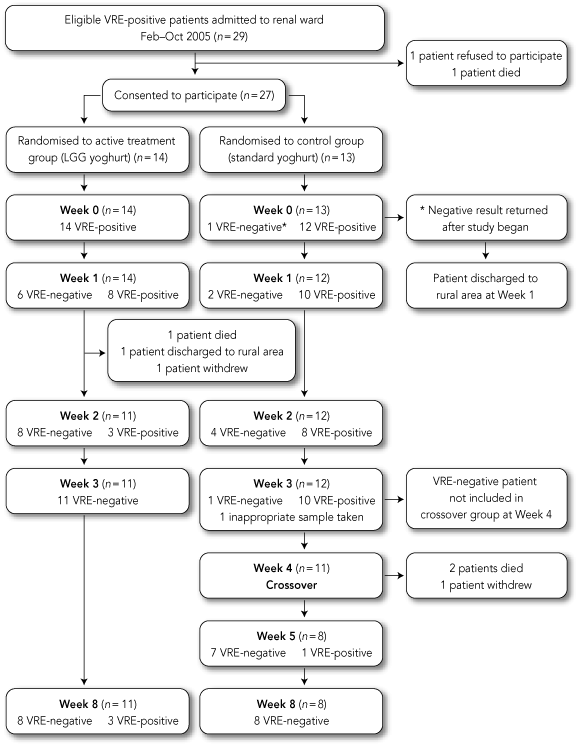
Twenty-nine VRE-positive patients were invited to enter the study (Box 1), of whom one refused and one ceased dialysis. Patient characteristics at entry were similar. The majority were receiving one or more antibiotics immediately before entry to the study (Box 2). All but two patients had renal failure. These two belonged to other medical units, but were treated on the renal ward. Compliance with treatment was excellent, although compromised by “nil orally” orders.
While all strains of lactobacilli are resistant to vancomycin, most do not survive stomach acid and duodenal bile acids in sufficient numbers to reach the bowel. However, live LGG bacteria can survive the stomach and bile acids to remain in sufficient numbers in the bowel. Thus, treatment with LGG has been proposed as a means of preventing overgrowth of bacteria that are resistant to the antibiotics used regularly in renal patients.9,10 The LGG strains are resistant to vancomycin but susceptible to a broad range of other antibiotics.
Potential mechanisms for the effectiveness of LGG in clearing intestinal infections include competitive colonisation, wherein LGG binds to the enteric epithelium and inhibits adhesion of pathogens such as Escherichia coli (a process that has been demonstrated in vitro).11 Secondly, LGG may possess antimicrobial activity against VRE, similar to that of L. ruminus SPM0211,12 although this has not been demonstrated. Thirdly, LGG may compete with VRE for consumption of monosaccharides, thereby slowing VRE growth. This mechanism has been shown to be partly responsible for the effectiveness of LGG against C. difficile.13 Finally, lactobacilli produce short-chain fatty acids that lower the colonic pH and favour the growth of less pathogenic organisms.
Studies have shown that no strains of Lactobaccillus possess the vanA, vanB or vanC gene, which provides some reassurance about the safety of LGG.5 However, the safety of the probiotic in patients who are extremely unwell or immunocompromised is uncertain. There were no adverse effects of LGG detected in our study, but Lactobacillus bacteraemia has been described in severely ill patients with cancer, gastrointestinal disease or liver disease.14,15
Received 5 September 2006, accepted 12 February 2007
- Karen J Manley1
- Margaret B Fraenkel2
- Barrie C Mayall3
- David A Power4
- Austin Health, Melbourne, VIC.
Our research was made possible by a grant from the Renal Society of Australasia. We wish to thank Associate Professor Paul Johnson and Dr Matthew Roberts, of Austin Health, for advice and statistical help.
None identified.
- 1. Zirakzadeh A, Patel R. Epidemiology and mechanisms of glycopeptide resistance in enterococci. Curr Opin Infect Dis 2005; 18: 507-512.
- 2. Willems RJ, Top J, van Santen M, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 2005; 11: 821-828.
- 3. Tenover FC, McDonald LC. Vancomycin-resistant staphylococci and enterococci: epidemiology and control. Curr Opin Infect Dis 2005; 18: 300-305.
- 4. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002; 8: 802-807.
- 5. Klein G, Hallmann C, Casas IA, et al. Exclusion of vanA, vanB and vanC type glycopeptide resistance in strains of Lactobacillus reuteri and Lactobacillus rhamnosus used as probiotics by polymerase chain reaction and hybridization methods. J Appl Microbiol 2000; 89: 815-824.
- 6. Klein G, Pack A, Reuter G. Antibiotic resistance patterns of enterococci and occurrence of vancomycin-resistant enterococci in raw minced beef and pork in Germany. Appl Environ Microbiol 1998; 64: 1825-1830.
- 7. Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 1987; 2: 1519.
- 8. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003; 47: 2492-2498.
- 9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 1999; 135: 564-568.
- 10. Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 2002; 97: 2744-2749.
- 11. Mack DR, Michail S, Wei S, et al. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 1999; 276: G941-G950.
- 12. Yun JH, Yim DS, Kang JY, et al. Identification of Lactobacillus ruminus SPM0211 isolated from healthy Koreans and its antimicrobial activity against some pathogens. Arch Pharm Res 2005; 28: 660-666.
- 13. Wilson KH, Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun 1988; 56: 2610-2614.
- 14. Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005; 115: 178-181.
- 15. Cannon JP, Lee TA, Bolanos JT, Danziger LH. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005; 24: 31-40.





Abstract
Objective: To determine whether eating Lactobacillus rhamnosus GG (LGG) in the form of commercially available yoghurt improves clearance of vancomycin-resistant enterococci (VRE).
Design: Double-blind, randomised, placebo-controlled trial.
Setting: Renal ward of Austin Health, a tertiary hospital, Feb–Oct 2005.
Participants: 27 VRE-positive patients, 14 receiving active treatment and 13 controls.
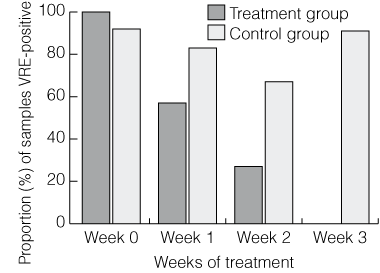
Interventions: Subjects were randomly assigned to either a treatment group (receiving 100 g daily of yoghurt containing LGG for 4 weeks) or a control group (receiving standard pasteurised yoghurt). Faecal samples were obtained three times at about weekly intervals. Treated patients were tested for VRE again at 8 weeks. Patients in the control group who had failed to clear VRE after 4 weeks were then given LGG-containing yoghurt for 4 weeks, as an open continuation.
Main outcome measure: Number of faecal specimens clear of VRE.
Results: Of the 27 patients enrolled, 23 completed the study. Two patients were lost to follow-up, one died and one withdrew. All 11 patients in the treatment group who completed the study cleared VRE. Three subjects reverted to VRE positivity after using antibiotics to which LGG is sensitive, while all others remained negative for at least 4 weeks after trial completion. Twelve control subjects completed the study, of whom one cleared VRE and 11 remained VRE-positive. Eight of these 11 patients were subsequently crossed over to receive LGG yoghurt, and all cleared VRE within 4 weeks.
Conclusion: To our knowledge, this is the first description of a probiotic therapy to successfully treat gastrointestinal carriage of VRE in renal patients. Further investigation of the use of LGG in VRE-positive patients is warranted.