Clinical record
Isolates were typed using a real-time polymerase chain reaction method based on single nucleotide polymorphisms (SNP) of the core genome and the presence or absence of variable genes, including the gene for Panton–Valentine leukocidin (pvl).1 All isolates had an SNP and variable gene profile characteristic of the Queensland clone (ST93-MRSA-IV) of community-associated MRSA (CA-MRSA), including the presence of pvl.
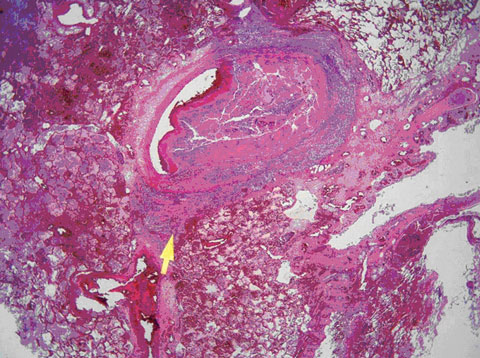
Postmortem examination showed that the principal pathology lay in the lungs and myocardium. The lungs showed multiple foci of bronchopneumonia, many coalescing to form extensive areas of lobar pneumonia. However, the most striking feature seen on histology was involvement of the pulmonary vasculature by staphylococcal septicaemia. Staphylococci had invaded the walls of multiple blood vessels, producing a florid vasculitis with subsequent secondary thrombosis of the involved vessels (Figure). This process involved both large and small vessels to such an extent that a lethal degree of bilateral arterial thrombosis had developed. The larger thrombosed vessels were obvious at macroscopic examination of lung slices. Multiple small thrombi were seen on microscopy. The myocardium showed focal abscesses containing staphylococcal colonies. Adjacent myocardial fibres showed necrosis, which correlated with the patient’s raised troponin level. The other organs of the body were remarkably free of sepsis, the spleen was normal, and the spinal column showed no evidence of osteomyelitis. A furuncle on the left elbow was confirmed.
Virulent strains of methicillin-resistant Staphylococcus aureus (MRSA) have recently emerged in community settings around the world (including many parts of Australia)2 and are causing community-acquired infection with increasing frequency.3 Most of the virulent strains carry the genes for producing Panton–Valentine leukocidin (PVL), a potent necrotising toxin. They most commonly cause primary skin and soft tissue infections such as furuncles and abscesses, but can also give rise to severe invasive conditions, including necrotising pneumonia.4 While uncommon, necrotising pneumonia is associated with a high mortality rate.
In Australia, two major strains of PVL-positive, community-associated MRSA (CA-MRSA) are currently circulating: the Queensland (QLD) clone and the south-west Pacific (SWP) clone. Currently, these strains predominate among CA-MRSA in Queensland, New South Wales and the Australian Capital Territory, while in other states, PVL-negative strains are more common.2
Lessons from practice
A history of recurrent furunculosis in a patient or in family members may precede severe Staphylococcus aureus sepsis, including necrotising pneumonia.
Patients with recurrent infection due to S. aureus should be tested for persistent nasal carriage. Treatment aimed at eradication could be considered.
The prevalence of virulent strains of community-associated methicillin-resistant S. aureus (CA-MRSA) is increasing in many parts of Australia. Knowledge of local prevalence would be valuable in guiding empirical treatment.
In communities where CA-MRSA is prevalent, suspected severe sepsis due to S. aureus should be treated with a combination of vancomycin and one of dicloxacillin, flucloxacillin or cephalothin until culture and susceptibility results are available.
Necrotising pneumonia due to PVL-positive S. aureus is often rapidly fatal, as in the case described here. A study by Gillet et al recorded a mortality rate of 37% within 48 hours of presentation.5 A significant association with preceding furunculosis was also noted. Most cases occurred in otherwise healthy children and young adults. A recently reported fatal case of CA-MRSA necrotising pneumonia in an Indigenous person was also in a previously healthy young adult.6 The patient in our case had a history of recurrent furunculosis and a furuncle on her elbow at presentation, both commonly caused by PVL-positive S. aureus. Two family members had also suffered from recurrent furunculosis. All isolates from the patient and from nose swabs of three family members belonged to the QLD clone.
QLD and SWP clones are frequently sensitive to numerous non-β-lactam antimicrobials.2 Agents such as clindamycin and cotrimoxazole may be used to treat mild-to-moderate CA-MRSA infections such as furunculosis, depending on the organism’s susceptibility.7 Agents that act against protein synthesis (and therefore toxin production) have a theoretical advantage in the treatment of toxin-related infectious syndromes, but good clinical studies in this area are lacking. The use of clindamycin for treating invasive CA-MRSA infections is supported by one retrospective study in children.8 Linezolid, a new agent also active against protein synthesis, has been shown to be superior to vancomycin, but only in complicated skin and soft tissue infections.9 Use of one of these agents, perhaps in combination with established anti-staphylococcal antibiotics, is worthy of prospective study. The current national recommendation for treating suspected MRSA pneumonia is to administer vancomycin together with a β-lactam antibiotic (dicloxacillin, flucloxacillin or cephalothin) until susceptibility data are known.7
- 1. Huygens F, Inman-Bamber J, Nimmo GR, et al. Staphylococcus aureus genotyping using novel real-time PCR formats. J Clin Microbiol 2006; 44: 3712-3719.
- 2. Nimmo GR, Coombs GW, Pearson PC, et al. Methicillin-resistant Staphylococcus aureus in the Australian community: an evolving epidemic. Med J Aust 2006; 184: 384-388. <MJA full text>
- 3. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin resistant Staphylococcus aureus carrying Panton–Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003; 9: 978-984.
- 4. Peleg AY, Munckhof WJ, Kleinschmidt SL, et al. Life-threatening community-acquired methicillin-resistant Staphylococcus aureus infection in Australia. Eur J Clin Microbiol Infect Dis 2005; 24: 384-387.
- 5. Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton–Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002; 359: 753-759.
- 6. Peleg AY, Munckhof WJ. Fatal necrotising pneumonia due to community-acquired methicillin-resistant Staphylococcus aureus (MRSA) [letter]. Med J Aust 2004; 181: 228-229. <MJA full text>
- 7. Antibiotic Expert Group. Therapeutic guidelines: antibiotic. 13th ed. Melbourne: Therapeutic Guidelines Limited, 2006.
- 8. Martinez-Aguilar G, Hammerman WA, Mason EO Jr, Kaplan SL. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J 2003; 22: 593-598.
- 9. Weigelt J, Itani K, Stevens D, et al. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2005; 49: 2260-2266.





We thank Flavia Huygens for her assistance with technical aspects of the typing assays.
None identified.